|
|
Energy changes in chemical reactions- thermochemistry
- Thermodynamic system, state function
- A thermodynamic system is just a fancy name for the system that you are studying.
- Isolated system: no exchange of heat, work, or matter with the surroundings.
- Closed system: exchange of heat and work, but not matter with the surroundings.
- Open system: exchange of heat, work and matter with the surroundings.
- A state function is path-independent and depends only on the initial and final states.
- State functions include: ΔH (enthalpy), ΔS (entropy), ΔG (free energy change), ΔU (internal energy change).
- State function is also called state quantity, or function of state.
- Conservation of energy
- The total energy of an isolated system remains constant.
- The total energy of a closed or open system plus the total energy of its surroundings is constant.
- Total energy is neither gained nor lost, it is merely transferred between the system and its surroundings.
- Endothermic/exothermic reactions
- Endothermic = energy is taken up by the reaction in the form of heat. ΔH is positive.
- Exothermic = energy is released by the reaction in the form of heat. ΔH is negative.
- enthalpy H and standard heats of reaction and formation
- enthalpy or H is the heat content of a reaction. Mnemonic: H stands for heat.
- ΔH is the change in the heat content of a reaction. + means heat is taken up, - means heat is released.
- Standard heat of reaction, ΔHrxn, is the change in heat content for any reaction.
- Standard heat of formation, ΔHf, is the change in heat content a formation reaction.
- A formation reaction is where a compound or molecule in its standard state is formed from its elemental components in their standard states. The standard state is where things are in their natural, lowest energy, state. For example, oxygen is O2 (diatomic gas) and carbon is C (solid graphite).
- The unit for enthalpy is in energy (J), or it can be expressed as energy per mol (J/mol).
- Hess' law of heat summation
- ΔHrxn = Δ(ΔHf) = sum of ΔHf (products) - sum of ΔHf (reactants)
- Bond dissociation energy as related to heats of formation
- Bond dissociation is the energy required to break bonds.
- ΔHrxn = Bond dissociation energy of all the bonds in reactants - bond dissociation energy of all the bonds in products
- ΔHrxn = Enthalpy of formation of all the bonds in products - Enthalpy of formation of all the bonds in reactants.
- Bond dissociation energy is positive because energy input is required to break bonds.
- The enthalpy of formation of bonds is negative because energy is released when bonds form.
- Measurement of heat changes (calorimetry), heat capacity, specific heat (specific heat of water = 4.184 J/g·k)
- Heat capacity = the amount of heat required to raise the temperature of something by 1 °C.
- Molar heat capacity = heat capacity per mol = J / mol·°C
- Specific heat (capacity) = heat capacity per mass = J / g·°C
- Celsius can be replaced by Kelvin here because a change in 1 °C is the same as a change in 1 K.
- It takes 4.2 J of heat energy to raise the temperature of 1 gram of water by 1 °C.
- Some useful conversion factors:
- 1 calorie = 4.2 J; 1 Calorie (with capital C) = 1000 calorie = 4200 J.
- For water, 1 gram = 1 cubic centimeter = 1 mL
- Entropy as a measure of "disorder"; relative entropy for gas, liquid, and crystal states
- Entropy = measure of disorder = energy / temperature = J / K (it can also be expressed as molar entropy in J / mol·K)
- Entropy of gas > liquid > crystal states.
- At room temperature, the gas molecules are flying around, but the table in front of you is just sitting there. So, gases have more disorder.
- Reactions that produces more mols of gas have a greater increase in entropy.
- Free energy G
- Free energy is the energy available that can be converted to do work.
- ΔG = ΔH - TΔS
- T is temperature in Kelvin.
- Spontaneous reactions and standard free energy change
- Spontaneous reactions are reactions that can occur all by itself.
- Spontaneous reactions have negative ΔG.
- Do not assume that an exothermic reaction is spontaneous, because a large, negative ΔS can cause it to become nonspontaneous.
- Do not assume that an endothermic reaction is nonspontaneous, because a large, positive ΔS can make it spontaneous.
- Do not assume that spontaneous reactions will occur quickly, because it may take a million years for it to happen, depending on its kinetics.
Thermodynamics
- Zeroth law (concept of temperature)
- 0th law of thermodynamics basically says that heat flows from hot objects to cold objects to achieve thermal equilibrium.
- Mathematically, if TA = TB, and TB = TC, then TA = TC. Where T is temperature.
- First law (ΔE = q + w, conservation of energy)
- 1st law of thermodynamics is based on the principle of conservation of energy, and it basically says that the change in total internal energy of a system is equal to the contributions from heat and work.
- ΔE is the same thing as ΔU, which is the change in internal energy.
- Q is the contribution from heat
- Q is positive when heat is absorbed into the system (ie. heating it).
- Q is negative when heat leaks out of the system (ie. cooling it).
- W is the contribution from work.
- W is positive when work is done on the system (ie. compression).
- W is negative when work is done by the system (ie. expansion).
- Equivalence of mechanical, chemical, electrical and thermal energy units
- If it's energy, it's Joules. It doesn't matter if it's potential energy, kinetic energy, or any energy - as long as it's energy, it has the unit Joules.
- Energy is equivalent even if they are in different forms. For example, 1 Joule of mechanical energy can be converted into 1 Joule of electrical energy (ignoring heat loss) - no more, no less.
- Second law: concept of entropy
- The 2nd law states that the things like to be in a state of higher entropy and disorder.
- An isolated system will increase in entropy over time.
- An open system can decrease in entropy, but only at the expense of a greater increase in entropy of its surroundings.
- The universe as a whole is increasing in entropy.
- ΔS ≥ q / T
- q is the heat transferred.
- T is the temperature in Kelvin.
- For reversible processes ΔS = q / T.
- For irreversible processes ΔS > q / T.
- Real processes that occur in the world are never reversible, so entropy change is always greater than the heat transfer over temperature.
- Because of the irreversibility nature of real processes, as long as anything occurs, the entropy of the universe increases.
- Temperature scales, conversion
|
K |
°C |
°F |
| Absolute zero |
0 |
-273 |
-460 |
| Freezing point of water / melting point of ice |
273 |
0 |
32 |
| Room temperature |
298 |
25 |
77 |
| Body temperature |
310 |
37 |
99 |
| Boiling point of water / condensation of steam |
373 |
100 |
212 |
- K = °C + 273
- F = °C x 1.8 + 32
- Heat transfer: conduction, convection, radiation
- Conduction: heat transfer by direct contact. Requires things to touch.
- Convection: heat transfer by flowing current. Need the physical flow of matter.
- Radiation: heat transfer by electromagnetic radiation (commonly in the infra-red frequency range). Does not need the physical flow of matter, can occur through a vacuum.
- Heat of fusion, heat of vaporization
- Also called latent heat of fusion, enthalpy of fusion and latent heat of vaporization, enthalpy of vaporization.
- Heat of fusion = ΔHfus = the energy input needed to melt something from the solid to the liquid at constant temperature.
- Heat of vaporization = ΔHvap = the energy input needed to vaporize something from the liquid to the gas at constant temperature.
- Latent heats can be expressed as molar values such as J / mol.
- The energy it takes to melt a solid is ΔHfus x #mols of that solid.
- The energy it takes to vaporize a liquid is ΔHvap x #mols of that liquid.
- Latent heats can also be expressed as J / mass, where energy can be obtained by multiplying the latent heats by the mass of the substance.
- Energy is released when either a gas condenses into a liquid, or when a liquid freezes into a solid. The energy released is the same as the energy of their reverse processes (see formula above).
- PV diagram: work done = area under or enclosed by curve
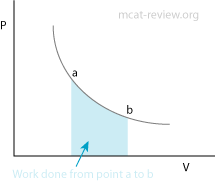
- PV diagrams depict thermodynamic processes by plotting pressure against volume.
- Adiabatic process: no heat exchange, q = 0. ΔE = W
- Isothermal process: no change in temperature ΔT = 0.
- Isobaric process: pressure is constant, W = PΔV.
- Isovolumetric (isochoric) process: volume is constant, W = 0. ΔE = q
- Calorimetry
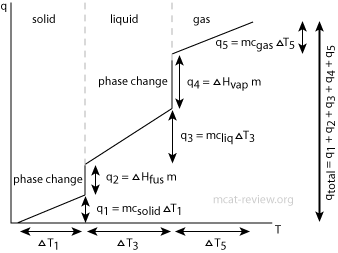
- q = mcΔT
- q is heat absorbed / heat input, m is mass, c is specific heat, and ΔT is change in temperature.
- This formula only works if no phase change is involved.
- Different phases have different specific heats, and on top of that, a phase change requires extra energy such as heat of fusion and heat of vaporization, which is why the above formula does not work across different phases.
- To work problems that involve a phase change, use the calorimetry equation individually for the different phases, then take into account of the heat of fusion or vaporization.
- For example, how much energy does it take to heat ice from -20 °C to water at 37 °C. There's 3 components to this question:
- For the ice phase from -20 °C to 0 °C, use q = mciceΔT, where ΔT is 20.
- For the phase transition, use heat of fusion: q = ΔHfus x #mols of ice/water, where ΔHfus is in energy per mol. (note: if the heat of fusion is given in energy per mass, then you should multiply it by the mass to get energy)
- For the water phase from 0 °C to 37 °C, use q = mcwaterΔT, where ΔT is 37.
Old topics
The topics below are outdated. They have been either modified or replaced by the most recent aamc publication.
- Measurement of heat changes (calorimetry); heat capacity; specific heat (specific heat of water = 1 cal per degrees Celsius)
- Heat capacity = the amount of heat required to raise the temperature of something by 1 °C.
- Molar heat capacity = heat capacity per mol = J / mol·°C
- Specific heat (capacity) = heat capacity per mass = J / g·°C
- Celsius can be replaced by Kelvin here because a change in 1 °C is the same as a change in 1 K.
- It takes 1 cal, or 4.2 J, of heat energy to raise the temperature of 1 gram of water by 1 °C.
- 1 calorie = 4.2 J; 1 Calorie (with capital C) = 1000 calorie = 4200 J.
- For water, 1 gram = 1 cubic centimeter = 1 mL
- First law: ΔE = Q - W (conservation of energy)
- 1st law of thermodynamics is based on the principle of conservation of energy, and it basically says that the change in total internal energy of a system is equal to the energy absorbed as heat minus the energy lost from doing work.
- ΔE = Q - W
- ΔE is the same thing as ΔU, which is change in internal energy.
- Q is the heat absorbed into the system.
- W is the work done by the system.
- An alternative expression for the first law is ΔE = Q + W, where work is either positive if done on the system, or negative if done by the system (this is the classical expression of ΔE = Q - W).
- Expansion = work done by the system -> ΔE = Q - W
- Compression = work done on the system -> ΔE = Q + W
- Specific heat, specific heat of water (1 cal / g·°C)
- 1 cal / g·°C = 4.2 J / g·°C = 0.001 Cal / g·°C = 4200 J / kg·°C = 4.2 kJ / kg·°C
|
|
