|
|
Absorption spectroscopy
- infrared region
- intramolecular vibrations and rotations
- Vibrations: bonds can stretch, compress and bend like a spring. It is this vibration that is measured in IR-spec.
- Rotations: molecules can rotate. Rotations produce waves mainly in the microwave region. However, part of the rotation spectra does overlap with the vibration spectra.
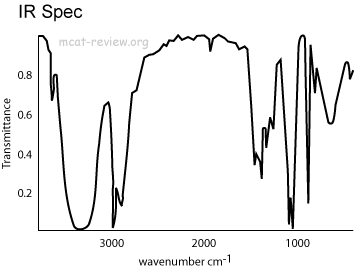
- Infrared spectra plots transmittance vs. wavenumbers, cm-1
- Transmittance increases as you go up the y-axis.
- Where transmittance dips down, that's a region of absorbance.
- Wavenumbers decrease from left to right.
- Wavenumbers are correlated to frequency.
- Peaks toward the left have higher frequency of vibration.
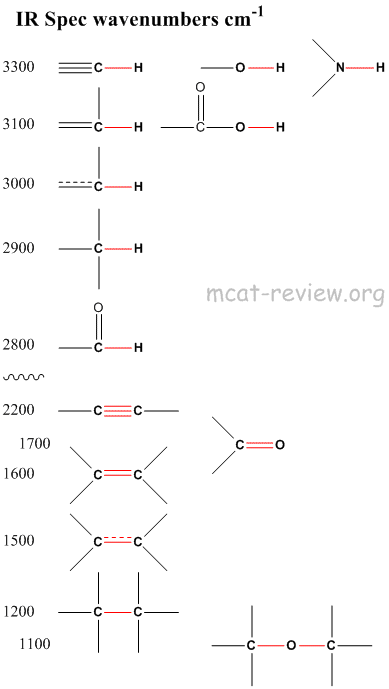
- recognizing common characteristic group absorptions, fingerprint region
- Anything around 3000 cm-1 involves a hydrogen atom, be it O-H, N-H, or C-H.
- Anything around 2000 cm-1 and below does not involve hydrogen, be it C=O, C=C, C-C, or C-O.
- With the same atoms, the higher the bond order, the faster it vibrates, and so the higher the wavenumber.
- 1700 cm-1 is for the carbonyl group. Remember this.
- 3300 cm-1 can be O-H, N-H, or alkyne C-H. OH is the broadest, N-H slightly sharper, alkyne C-H is very sharp.
- Broad peaks are due to hydrogen bonding (OH and NH).
- Below 1300 cm-1 is called the fingerprint region.
- Patterns in the fingerprint region are unique for each compound just like a fingerprint is unique for each person.
- visible region
- absorption in visible region gives complementary color (e.g., carotene)
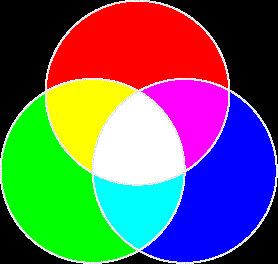
- There are primary colors of light and primary colors of pigment.
- Primary colors of light
- Primary colors of pigments
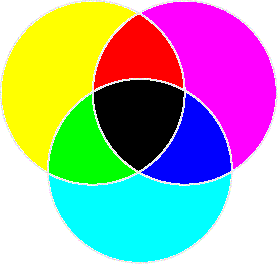
- Complementary color is the color that's on the opposite side of the color wheel. For example, the complementary of red is cyan.
- The absence of (when you absorb) a primary color of light, you end up with its complementary color.
- The primary colors of pigments is exactly the complementary colors of the primary colors of light. This is because pigments absorb a certain color of light and reflect the rest back into your eyes.
- Carotene absorbs blue light and reflect the others into your eyes. The absence of blue produces yellow, the complementary color of blue.
- effect of structural changes on absorption (e.g., indicators)
- Changes to chemical structure can lead to changes in absorption.
- H-indicator <--> H+ + Indicator-
- H-indicator absorbs at a certain wavelength and is of one color.
- Indicator- absorbs at a different wavelength so is of a different color.
- At low pH, high [H+], H-indicator and its color will predominate.
- At high pH, low [H+], indicator- and its color will predominate.
- At neutral pH, both H-indicator and indicator- will co-exist in an equilibrium, so the color will be a mixture of the two.
- You should know the colors of the universal indicator:
- Red: very acidic
- Orange: acidic
- Yellow: weakly acidic
- Green: neutral
- Blue: basic
- Purple: very basic
- ultraviolet region
- pi-electron and non-bonding electron transition
- Every time you have a bond, the atoms in a bond have their atomic orbitals merged together to form molecular orbitals.
- Every time you have molecular orbitals, you get bonding molecular orbitals and non-bonding and/or anti-bonding orbitals.
- Normally, electrons sit in their bonding orbitals because it is the most stable there. If bonding orbitals are full, then non-bonding orbitals are occupied.
- Given enough energy (as in absorption), the electrons transition from the bonding or non-bonding orbitals to the anti-bonding orbitals.
- If too much energy is absorbed, enough electrons escape the bonding orbitals / enter the anti-bonding orbitals to break the bond completely.
- For UV absorption, we're not worried about breaking bonds. We're only interested in the pi-electrons of double bonds because their molecular orbital transitions result in UV absorption.
- Double bonds absorb UV because the pi electrons transition from the bonding and non-bonding molecular orbitals to the anti-bonding orbitals.
- conjugated systems
- Conjugated systems decreases the energy of electromagnetic radiation that is absorbed.
- The more conjugated double bonds there are, the longer the wavelengths of absorbed radiation.
- If there are enough conjugated double bonds, the molecule will start to absorb in the visible region.
Mass spectrometry: m/e ratio, parent peak
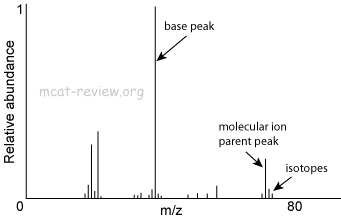
- Mass spec is when you bombard a molecule with electrons.
- When electrons smash into your molecule, it is fragmented into ions.
- What if the electrons "miss" your molecule? Ans: Your molecule is neither fragmented nor ionized. Uncharged molecules are not detected and are not included in the mass spectra.
- What if the electrons do not break apart your molecule, but merely ionizes it? Ans: this "molecular ion" will be detected as the parent peak, also called the molecular ion peak.
- What if the electrons not only ionize but also fragment your molecule? Ans: all the fragmented ions will be detected and plotted in the mass spectra.
- The faster (higher energy) the bombarding electron, the more fragmentation.
- The more fragmentation, the smaller the molecular ion peak.
- These ions have a characteristic mass to charge ratio (m/e or m/z).
- A magnetic field resolves (separates) the different m/z ions so they can be individually detected and plotted on a spectrum.
- The resulting spectrum plots Relative abundance vs. the m/z ratio.
- The parent peak, or the molecular ion peak, is the peak that depicts the ion of the molecule without fragmentation. It has the highest m/z ratio.
- Peaks clustered really close to one another depicts isotopes.
- The base peak is the tallest peak (most abundant species).
- Mass spec is useful for:
- Measuring the molecular weight of a molecule.
- Identify the molecule by fragmentation patterns.
- Identity heteroatoms by their characteristic isotope ratios.
NMR spectroscopy
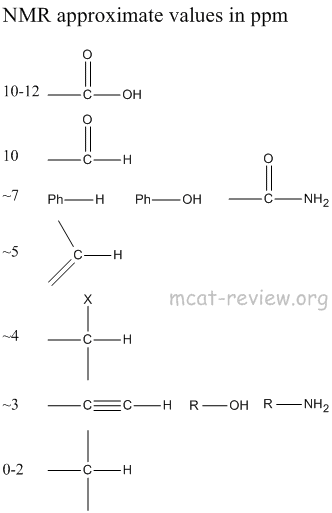
- protons in a magnetic field; equivalent protons
- Protons have spins of up or down (+½ or -½, counterclockwise or clockwise. The detailed vectors are not important here, so simply up or down is fine).
- With an even number of protons, the spins pair up and the up and down spins of all the protons cancel each other out.
- With an odd number of protons, there is a net spin of up or down.
- Normally, both up or down spins are equal in energy (they are degenerate). So, either way goes.
- In the presence of a magnetic field, the spin that lines up with the magnetic field gets the lower energy. If the external magnetic field is up, then you better spin up. If the magnetic field is down, then you better spin down.
- If we were to give the protons some energy (by radio wave absorption), then the protons can be promoted (flipped) to the higher energy spin, which is opposite to the direction of the external magnetic field. This absorption is called resonance. The resonance frequency is the frequency of the radio wave that's needed to cause a flip in spin.
- The resonance frequency (or energy or field strength) of absorption is called the chemical shift.
- Different protons have different resonance frequencies.
- Equivalent protons have the same resonance frequencies.
- You can substitute X at any of the equivalent protons, and you should end up with the same new compound. If not, then they're not equivalent protons.
- What makes protons have different resonance frequencies depends on what atom they're close to.
- NMR measures the chemical shift relative to a standard called TMS (tetramethylsilane) in unit of ppm.
- The more "different" two protons are, the farther their chemical shifts
- What makes protons "different" is the degree of electron shielding or deshielding.
- Next to stuff like carbon, hydrogen is shielded by electrons because carbon is not so electronegative.
- Next to stuff like oxygen, hydrogen is deshielded because oxygen is very electronegative.
- When things are shielded, the magnetic field is smaller and they have small chemical shifts and appear upfield (to the right).
- When things are deshielded, the magnetic field is larger and they have large chemical shifts and appear downfield (to the left).
- NMR is nuclear magnetic resonance because the nuclear stands for protons; magnetic stands for the external magnetic field; the resonance stands for the absorption of radio waves.
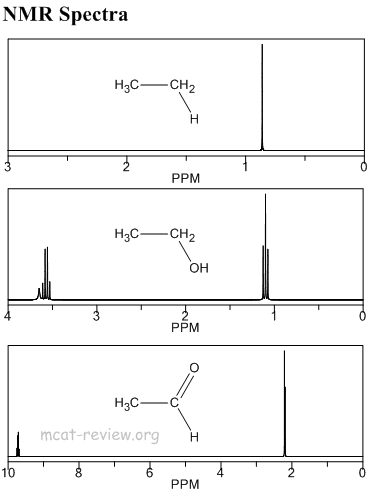
- Signals by n equivalent protons add up to produce one signal the height n times the signal for a single proton.
- spin-spin splitting
- Magnetic fields produced by neighboring protons cause spin-spin splitting.
- Neighboring is defined as 3 bonds away, which is the same thing as hydrogens attached to adjacent atoms.
- Things are split into n+1 peaks, where n is the number of neighboring protons.
- Aromatic protons can split over 3 bonds, which is why the NMR spectra for the aromatic region is a mess.
- The J value defines how far apart things get split.
- Protons across single and aromatic bonds get split approximately the same.
- Protons across double bonds get split farther apart.
|
|
