|
|
Enzyme structure and function
- Function of enzymes in catalyzing biological reactions
- Enzymes are catalysts, which are things that increase the rate of a reaction, but does not get used up during the reaction.
- Structure determines function. A change in structure => a change in function.
- Important biological reactions catalyzed by enzymes:
- Metabolism
- DNA synthesis
- RNA synthesis
- Protein synthesis
- Digestion
- Reduction of activation energy
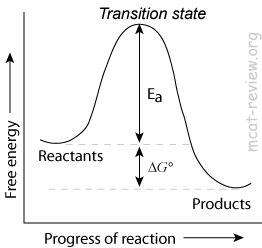 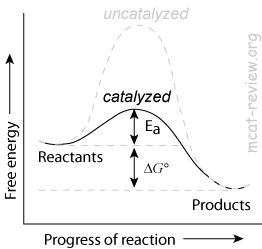
- Enzymes decrease the activation energy (Ea) of a reaction by lowering the energy of the transition state.
- Enzymes increase the rate of a reaction by decreasing the activation energy.
- Enzymes will increase the rate constant, k, for the equation rate = k[A][B].
- Enzymes do NOT change the Keq of a reaction.
- Enzymes do not change Keq because it lowers the activation energy for BOTH forward and reverse reactions.
- Enzymes will make the reverse reaction go faster also.
- Enzymes do not change ΔG, the net change in free energy.
- Enzymes affect the kinetics of a reaction, but not the thermodynamics.
- Substrates and enzyme specificity
- Enzyme-substrate interactions occur at the enzyme's active site.
- Enzyme-substrate specificity derives from structural interactions.
- Lock and key model: rigid active site. Substrate fits inside the rigid active site like a key.
- Induced fit model: flexible active site. Substrate fits inside the flexible active site, which is then induced to "grasp" the substrate in a better fit.
- Enzymes can be specific enough to distinguish between stereoisomers.
- Enzymes can be protein or RNA.
- Almost all enzymes in your body is made of protein.
- The most important RNA enzyme in your body is the ribosome.
- Enzyme structure derives from 4 levels.
- Primary: this is the sequence of the protein or RNA chain.
- Secondary: this is hydrogen bonding between the protein backbone. Examples include alpha helices and beta sheets (backbone H-bonding). For RNA, this is base pairing.
- Tertiary: this is the 3-D structure of the enzyme. This involves -R group interactions and spatial arrangement of secondary structure.
- Quaternary: when more than 1 chain is involved. When you hear about "dimers", "trimers", "tetramers", "oligomers", that's quaternary structure.
- Heat and extreme pH denatures enzymes by altering their structure.
Control of enzyme activity
- Feedback inhibition
- The product of a pathway inhibits the pathway.
- For example, hexokinase, the first enzyme in glycolysis, is inhibited by its product glucose-6-phosphate.
- Competitive inhibition
- An inhibitor competes with the substrate for binding to the active site.
- Competitive inhibition increases the amount of substrate needed to achieve maximum rate of catalysis.
- Competitive inhibition does NOT change the maximum possible rate of the enzyme's catalysis.
- You can overcome competitive inhibition by providing more substrate.
- Non-competitive inhibition
- An inhibitor binds to an allosteric site on the enzyme to deactivate it.
- The substrate still have access the active site, but the enzyme is no longer able to catalyze the reaction as long as the inhibitor remains bound.
- Non-competitive inhibition decreases the maximum possible rate of the enzyme's catalysis.
- Non-competitive inhibition does NOT change the amount of substrate needed to achieve the maximum rate of catalysis.
- You can't overcome non-competitive inhibition by adding more substrate.
Basic metabolism
- Metabolism consists of two parts: Catabolism and anabolism.
- Catabolism is breaking stuff down for energy. This is the part that the MCAT (and what we) focuses on.
- Anabolism is using energy to build stuff for storage.
- Unless otherwise stated, everything here on metabolism is about catabolism - breaking things down for energy.
- Another name for metabolism is cellular respiration.
- Steps of aerobic metabolism (needs oxygen)
- Glycolysis
- Oxidative decarboxylation
- Krebs cycle
- Electron transport chain.
- Steps of anaerobic metabolism (don't need oxygen)
- Glycolysis
- Alcohol or lactic acid fermentation
- Aerobic metabolism of glucose
- Complete oxidation of metabolite (glucose) to carbon dioxide.
- ~30 ATP produced per glucose.
- C6H12O6 + 6O2 => 6CO2 + 6H2O
- C6H12O6: this is glucose. You get it from your diet.
- 6O2: this is molecular oxygen that you breathe in.
- 6CO2: this is carbon dioxide produced by the Krebs cycle. Both the carbon and oxygen in this CO2 comes from the metabolite (glucose).
- 6H2O: this is water produced in the electron transport chain. The oxygen comes completely from the molecular oxygen that you breathe in.
- If we were to follow the carbon in the metabolite (glucose), it will end up in carbon dioxide.
- If we were to follow the oxygen in the metabolite (glucose), it will end up in carbon dioxide.
- If we were to follow the oxygen you breathe in, it will end up in water.
- As for the hydrogens, they'll either be in water, exist as protons in solution, or be transferred to some other entity.
- As we can see, the total reaction involves complete oxidation of the metabolite (glucose) and complete reduction of molecular oxygen.
- When electrons pass from the metabolite (glucose) to molecular oxygen, energy is released.
- The electron transport chain harnesses this energy.
- Anaerobic metabolism of glucose
- Partial oxidation of metabolite (glucose) to pyruvate.
- 2 net ATP produced per glucose.
- Pyruvate is then reduced to either alcohol or lactate.
- Bacteria reduce pyruvate to alcohol in a process called alcohol fermentation.
- Humans reduce pyruvate to lactate in a process called lactic acid fermentation.
- Glycolysis, anaerobic and aerobic, substrates and products
- Glycolysis = convert glucose (6 carbons) to 2 molecules of pyruvate (3 carbons).
- Location: cytosol.
- 2 net ATP made for every glucose (2 input ATP, 4 output ATP).
- 2 NADH made for every glucose.
- Occurs under both aerobic and anaerobic conditions.
- Glycolysis is inhibited by ATP.
- Aerobic decarboxylation (mitochondrial matrix) = convert pyruvate (3 carbons) to an acetyl group (2 carbons).
- 1 NADH made for every pyruvate.
- Only occurs in the presence of oxygen.
- Acetyl group attaches to Coenzyme A to make acetyl CoA.
- Anaerobic fermentation (cytosol) = redox reaction: reduce pyruvate, oxidize NADH.
- 1 NAD+ made for every pyruvate.
- Alcohol fermentation = pyruvate reduced to ethanol.
- Lactic acid fermentation = pyruvate reduced to lactate.
- The purpose of anaerobic fermentation is to regenerate NAD+, which is needed for glycolysis.
- Krebs cycle, substrates and products, general features of the pathway
- Location: matrix of mitochondria.
- Acetyl CoA feeds into the cycle.
- 3 NADH made per acetyl CoA.
- 1 FADH2 made per acetyl CoA.
- 1 ATP (GTP) made per acetyl CoA.
- Coenzyme A is regenerated (during the first step of the cycle).
- Krebs cycle, TCA, Tricarboxylic acid cycle, citric acid cycle all mean the same thing.
- Krebs cycle is Inhibited by ATP and NADH.
- Electron transport chain and oxidative phosphorylation, substrates and products, general features of the pathway
- Location: the cristae (inner membrane of mitochondria).
- Input NADH
- Proton gradient
- The electron transport chain (ETC) is essentially a series of redox reactions, where NADH gets oxidized to NAD+ and O2 gets reduced to H2O.
- The series of redox reactions consists of electrons passing from NADH to FMN, to Coenzyme Q, iron-sulfur complexes, and cytochromes (cytochrome b, c and aa3) before finally being used to reduce oxygen.
- NADH is highest in energy, while O2 is lowest in energy. When electrons are passed from NADH down a series of proteins and finally to O2, energy is released.
- FADH2 is lower in energy than NADH, that's why it releases less energy when it gets oxidized.
- FADH2 skips FMN and passes its electrons to Coenzyme Q.
- The energy released from these reactions generates a proton gradient, which drives ATP synthase to make ATP. This is called oxidative phosphorylation.
- Proton gradient
- The energy released from passing electrons down the ETC is used to pump protons into the intermembrane space of the mitochondria.
- H+ concentration is very high in the intermembrane space (higher than those in the matrix). Thus, this establishes an electrochemical gradient called the proton gradient.
- H+ wants to migrate down the proton gradient (from the intermembrane space back into the matrix), but it can only do this by going through the ATP synthase.
- Like a water mill, ATP synthase harnesses the energy of the falling protons to convert ADP into ATP.
- The ETC is inhibited by certain antibiotics, by cyanide, azide, and carbon monoxide.
- Metabolism of fats and proteins
- Fat metabolism
- Location: beta-oxidation occurs in the matrix of the mitochondria. Ester hydrolysis occurs in the cytosol.
- Fatty esters gets hydrolyzed into free fatty acids by lipases.
- For example, triacylglycerol gets hydrolyzed into free fatty acids and glycerol.
- With the help of ATP, the fatty acid is "activated" at the acid end by CoA (to be precise, it turns into a thioester).
- A process called beta-oxidation breaks down the fatty-CoA, 2 carbons at a time, to make acetyl CoA.
- β-oxidation produces acetyl CoA and also FADH2 and NADH.
- The acetyl CoA feeds into the Krebs cycle, and the FADH2 and NADH feed into the ETC.
- On a per gram basis, fats give more energy than any other food source.
- Protein metabolism
- Proteins are broken down into amino acids by peptidases.
- The nitrogen in the amino acid is converted to urea (for desert animals, birds and reptiles, it is uric acid).
- The carbon in the amino acid is converted to pyruvate or acetyl-CoA, (or other metabolical intermediates such as oxaloacetate), depending on what amino acid it is.
- The carbon products from amino acid metabolism can either feed into the Krebs cycle, or be the starting material for gluconeogenesis.
|
|
