|
|
Electronic structure
- Orbital structure of hydrogen atom, principal quantum number n, number of electrons per orbital
- In the Bohr model, the hydrogen electron orbits the nucleus.
- In quantum mechanics, hydrogen electron exists in a spherical probability cloud around the nucleus.
- The principle quantum number, n, defines what shell the electron is in.
- n values start from one: 1,2,3 ...etc.
- Higher n shells are higher in energy (if subshells are the same).
- There are n squared orbitals per shell.
- There are 2 electrons per orbital.
- Thus, there are 2n^2 electrons per shell.
- Ground state, excited states
- Electrons are normally in their ground state.
- When they absorb energy, they get promoted to excited states.
- Excited states are higher in energy than ground states.
- Excited state electrons come back down to the ground state via release of energy.
- Absorption and emission spectra
- The absorption spectrum shows what wavelengths of light are absorbed.
- The absorption spectrum looks like black lines on a rainbow background.
- The emission spectrum shows what wavelengths of light are emitted.
- The emission spectrum looks like colored lines on a black background.
- The absorption spectrum corresponds to the emission spectrum in pattern.
- The emission spectrum shifts to a slightly longer wavelength.
- Quantum numbers l, m, s, and number of quantum states (electrons) per orbital
- l is the angular momentum quantum number: l are integers that range from 0 to n-1.
- spdf: l=0,1,2,3 for s,p,d,f respectively.
- spdf designates subshells.
- s subshells hold 1 orbital. p holds 3, d holds 5, f holds 7.
- each orbital holds a maximum of 2 electrons.
- s subshells hold a maximum of 1x2=2 electrons, p: 3x2=6, d: 5x2=10, f: 7x2=14.
- A generalized formula for the above pattern: for any subshell, 4l+2 electrons can be held.
- for a given shell, higher subshells have higher energy.
- a low shell with a high subshell may be higher in energy than a higher shell with a low subshell.
- m is the magnetic quantum number: m are integers that range from -l to l, including zero.
- s is the spin quantum number: s is either +1/2 or -1/2.
- Common names and geometric shapes for orbitals s, p, d
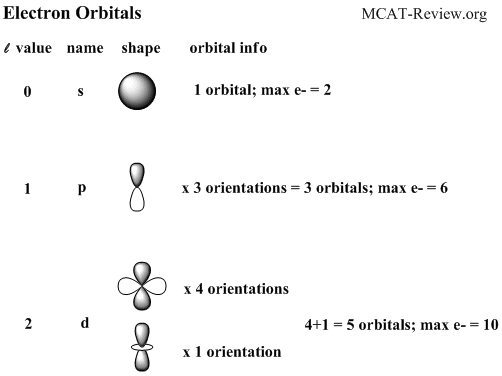
- Electrons are filled by occupying the lowest energy subshells first.
- Subshell arranged in increasing energy: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d
- The best way to memorize the above is by interpreting the periodic table:
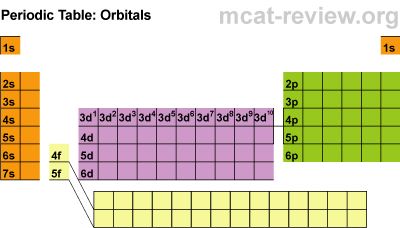
- Starting from the first row, going across, both hydrogen and helium is 1s. Next row: 2s then 2p. Third row: 3s then 3p. Fourth row: 4s, 3d, then 4p. Fifth row: 5s, 4d, then 5p. Sixth row: 6s, 4f, 5d, then 6p. Last row: 7s, 5f, then 6d. The pattern we get from looking at the periodic table is exactly in the order of increasing energy.
- For a given subshell, the columns represent how many electrons are in that subshell. For example, the fifth column of the d subshells contain elements that have 5 electrons in that subshell.
- The number of columns for each subshell indicate the maximum number of electrons that subshell can hold. For example, the d subshells have 10 columns showing that d orbitals can hold 10 electrons total.
- Conventional notation for electronic structure
- Conventional notation:
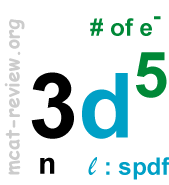
- Orbital diagrams:
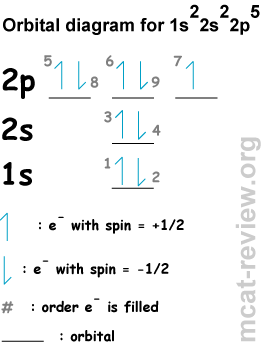
- Aufbau principle: shells / subshells of lower energy gets filled first (This is the most obvious rule. For example, 1s fills first, then 2s, then 2p ...etc. Review the exact order of energies because later on, the d subshells get filled after the s.
- Hund's rule: when you fill a subshell with more than 1 orbital (p, d, f), you first fill each orbital with a single electron and with the same spin (check out electrons 5, 6, and 7 in the orbital diagram, which fills according to Hund's rule). The reason for Hund's rule is that electron-electron repulsion in doubly occupied orbitals make them higher in energy than singly occupied orbitals.
- Pauli exclusion principle: 2 electrons in the same orbital must be of different spins (for example, check out electrons 5 and 8 in the orbital diagram).
- Watch out for d4 and d9 elements. Instead of s2d4 and s2d9, it's s1d5 and s1d10 because they want to achieve a half-full or full d subshell.
- Bohr atom
- Effective nuclear charge
- Effective nuclear charge = nuclear charge - shielding electrons.
- Shielding electrons are those that stand between the nucleus and the electron we are interested in.
- Shielding electrons are those that are in subshells closer to the nucleus (lower in energy) than the electron we are interested in.
- MCAT questions usually give you a diagram of the Bohr model, in which case, shielding electrons are those that orbits at a smaller radius.
- The higher the effective nuclear charge for an electron, the more stable it is (higher ionization energy, not easily knocked off).
- Effective nuclear charge increases for outer electrons as you go across (left to right) the periodic table.
The periodic table: classification of elements into groups by electronic structure; physical and chemical properties of elements
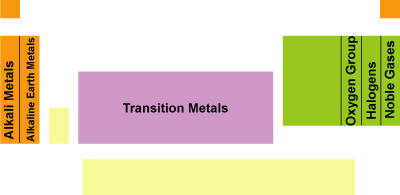
- Alkali metals
- Single valence electron - low ionization energy, very reactive.
- Wants to lose that electron to achieve empty valence shell.
- More reactive as you go down because of increasing radii.
- Reacts with oxygen to form oxides.
- Reacts with water to form hydroxides and releases hydrogen.
- Reacts with acids to form salts and releases hydrogen.
- Most commonly found in the +1 oxidation state.
- Alkaline earth metals
- 2 valence electrons - relatively low in ionization energy, quite reactive.
- Wants to lose both electrons to achieve empty valence shell.
- More reactive as you go down because of increasing radii.
- Reacts with oxygen to form oxides.
- Reacts with water to form hydroxides and releases hydrogen.
- Reacts with acids to form salts and releases hydrogen.
- Most commonly found in the +2 oxidation state.
- Halogens
- 7 valence electrons (2 from s subshell and 5 from p subshell) - high electron affinity, very reactive.
- Wants to gain one electron to achieve full valence shell.
- More reactive as you go up because of decreasing radii.
- Reacts with alkali metals and alkaline earth metals to form salts.
- Most commonly found in the -1 oxidation state.
- Noble gases
- Full valence shell of 8 - high ionization energy couple with low electron affinity.
- Don't react.
- Found in the oxidation state of 0.
- Transition metals
- High conductivity due to free flowing (loosely bound) outer d electrons.
- In the presence of ligands (when in a chemical complex), the d orbitals become nondegenerate (different in energy).
- Electron transitions between nondegenerate d orbitals gives transition metal complexes vivid colors.
- Varied oxidation states - but always +.
- Representative elements
- Representative elements include the s block and the p block of the periodic table.
- No free flowing (loosely bound) outer d electrons.
- Valence shell fills from left (1 electron) to right (8 electrons).
- Standard nomenclature from left to right: I A, II A, III A, IV A, V A, VI A, VII A, VIII A.
- Metals and non-metals
- Metals are to the left of metalloids.
- Non-metals are to the right of metalloids.
- Metalloids: diagonal line from Boron to Polonium: B, Si, As, Te, Ge, Sb, (Po).
| Chemical properties |
| Metals | Non-metals |
| Likes to lose electrons to gain a + oxidation state (good reducing agent). |
Likes to gain electrons to form a - oxidation state (good oxidizing agent). |
| Lower electronegativity - partially positive in a covalent bond with non-metal. |
Higher electronegativity - partially negative in a covalent bond with metal. |
| Forms basic oxides. |
Forms acidic oxides. |
| Physical properties |
| Good conductor of heat and electricity |
Poor conductor of heat and electricity |
| Malleable, ductile, luster, solid at room temp(except Hg) |
Solid, liquid, or gas at room temp. Brittle if solid and without luster. |
- Oxygen group
- The group (column) that contains oxygen.
- Oxygen and sulfur are chemically similar (if a question asks you what element you can substitute for oxygen and still keep the same chemical reactivity, then choose sulfur).
- Se - Te - Po = non-metal - metalloid - metal (or metalloid).
The periodic table: variations of chemical properties with group and row
- Electronic structure ...a repeat of electronic structure section above
- representative elements
- Representative elements include the s block and the p block of the periodic table.
- No free flowing (loosely bound) outer d electrons.
- Valence shell fills from left (1 electron) to right (8 electrons).
- Standard nomenclature from left to right: I A, II A, III A, IV A, V A, VI A, VII A, VIII A.
- noble gases
- Full valence shell of 8 - high ionization energy couple with low electron affinity.
- Don't react.
- Found in the oxidation state of 0.
- transition metals
- High conductivity due to free flowing (loosely bound) outer d electrons.
- In the presence of ligands (when in a chemical complex), the d orbitals become nondegenerate (different in energy).
- Electron transitions between nondegenerate d orbitals gives transition metal complexes vivid colors.
- Varied oxidation states - but always +.
- Valence electrons
- Electrons in the outer shell.
- Ranges from 1 to 8 from left to right of the representative elements.
- The valence electron rule does not apply to transition metals.
- First and second ionization energies
- definition of first ionization energy: The energy needed to knock off the first valence electron.
- definition of second ionization energy: The energy needed to knock off the second valence electron.
- prediction from electronic structure for elements in different groups or rows
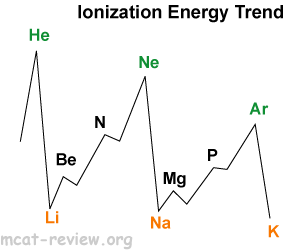
- Ionization energy decreases as you go down because of increasing radii.
- Ionization energy increases as you go right because of decreasing radii.
- Highest peaks are noble gases.
- Lowest troughs are alkali metals.
- Local maxima occurs for filled subshells and half-filled p subshells.
- Second ionization energy is always higher than the first ionization energy (usually a lot higher).
- Alkali metals and hydrogen: first ionization energy very low. Second ionization much higher.
- Alkaline earth metals: first ionization energy low. Second ionization energy also low.
- Electron affinity
- definition - electron affinity is the amount of energy released when something gains an electron (how easily it can gain an electron).
- variation with group and row
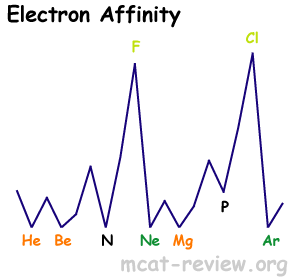
- As you go down a group, electron affinity decreases because of larger radii.
- As you go across (left to right) a row, electron affinity increases.
- Highest peaks are for the halogens.
- Lowest for noble gases.
- Local minima occurs for filled subshells and half-filled p subshells.
- Electronegativity
- definition - electronegativity is how much something hoards electrons in a covalent bond.
- comparative values for some representative elements and important groups
- Electronegativity increases toward the top right.
- Fluorine is the most electronegative element.
- Things around fluorine are highly electronegative: N, O, F, Cl, Br.
- Halogens are electronegative, especially toward the top of the group.
- Noble gases can be very electronegative if they participate in bond formation (Kr and Xe).
- Non-metals are more electronegative than metals.
- Covalent bond is a sharing of electrons between elements.
- The more electronegative element in a covalent bond gets a larger share of the electrons and has a partial negative charge
- The less electronegative (more electropositive) element in a covalent bond gets a smaller share of the electrons and has a partial positive charge.
- If the electronegativity difference is too great, an ionic bond occurs instead of a covalent one.
- Ionic bonds result from a complete transfer of electrons from the electropositive element to the electronegative element.
- Electron shells and the sizes of atoms
- Electron shells
- Electron shells are defined by the principle quantum number - the n value.
- Going down the periodic table means jumping to the next shell.
- As you fill to the next shell (Ne to Na), the effective nuclear charge decreases because the old shell stands in between the nucleus and the new shell.
- Filling to the next shell causes a jump in atom size because of decreased effective nuclear charge.
- As you go down a group (Na to K), the atomic size increases even though the effective nuclear charge stays the same, because higher shells have a larger radius than lower shells.
- Going across the periodic table means filling up the same shell (by going through subshells).
- As you fill up a shell, the effective nuclear charge increases because the atomic number (protons) is increasing while the same-shell electrons you add do not shield one another.
- With increasing effective nuclear charge, the electrostatic attraction (F=kQq/r^2) between the nucleus and the electrons increases, so the atom becomes more compact.
- The increasing effective nuclear charge and electrostatic attraction is why going across a periodic table means decreasing atomic size.
- Sizes of atoms
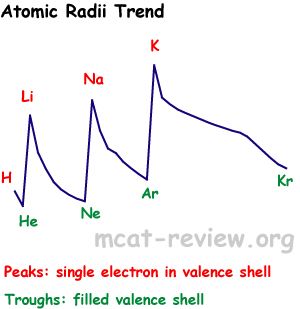
- Size increases as you go down a column.
- Size decreases as you go across (to the right of) a row.
- Atomic sizes may overlap if you zigzag on the periodic table.
|
|
