|
|
- Description
- nomenclature
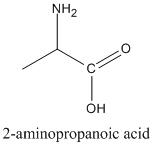
- Prefix: amino-
- For example, 2-aminopropanoic acid.
- Suffix: -amine.
- For example, propanamine.

- stereochemistry and physical properties
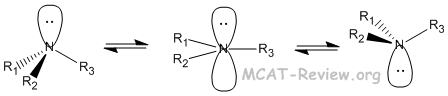
- 3° amines can be chiral. But they are always racemic because of spontaneous inversions at room temperature.
- Even protonated 3° amines undergo inversion because the proton comes on and off in an acid-base equilibrium.
- 4° amines can be chiral and they stay chiral because they don't undergo inversion.
- infrared absorption
- primary amines = R-NH2 = 2 N-H bonds = 2 peaks around 3300 cm-1.
- secondary amines = R2-NH = 1 N-H bonds = 1 peak around 3300 cm-1.
- tertiary amines = R3-N = no N-H bonds = 0 peak around 3300 cm-1.
- Major reactions
- amide formation
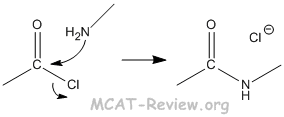
- Amine + acid derivative with a good leaving group → amide
- Usually the acide derivative is acyl chloride with chlorine as the leaving group. However, any other good leaving group will work.
- An important biological amide formation is the peptide bond formation in protein synthesis. Here amine + carboxylic acid → amide. The leaving group is water (not OH-).
- reactions with nitrous acid
- Ar-NH2 + HONO → Ar-N2+ + H2O + OH-
- nitrous acid = HNO2 = HONO
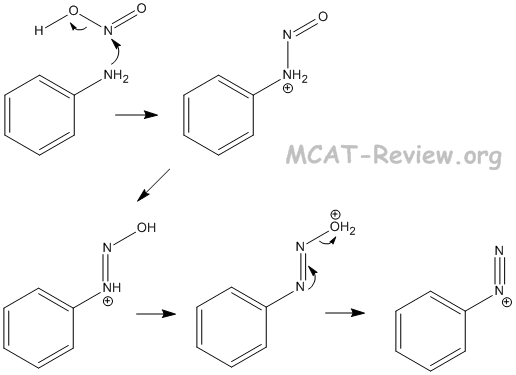
- The reason why the nitrogen in nitrous acid can be attacked is the following:
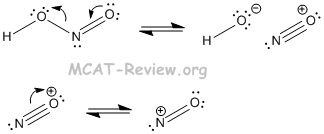
- HONO → NO+ + OH-
- The NO+ species is the strong electrophile.
- alkylation
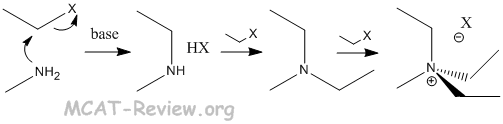
- multiple products formed from polyalkylation.
- Hoffman elimination (Hofmann elimination)
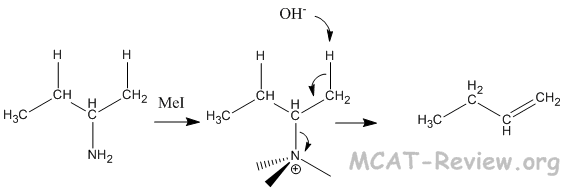
- amine + methyl iodide → exhaustive methylation of the amine → elimination with the methylated amine as leaving group.
- Unlike E1 reactions where the more substituted double bond is formed (Zaitsev), Hofmann elimination forms the less substituted double bond (Hofmann).
- A quick review of regiochemistry:
- E1 = zaitsev
- E2 with bulky base = Hofmann
- Hofmann elimination = Hofmann
- Hoffman and Hofmann mean the same thing.
- General principles
- basicity
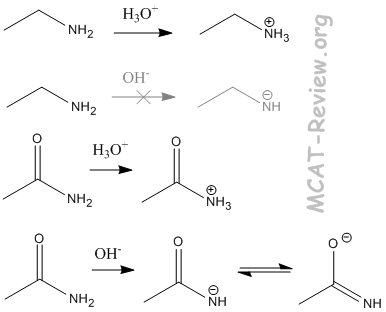
- Amines are basic. They like to gain a proton. R-NH2 → R-NH3+
- It is very difficult for neutral amines to lose a proton.
- An amide, however, can lose a proton much more easily. This is because the carbonyl group next to the nitrogen contributes to a resonance structure that places the negative charge on the oxygen. Thus, the negative charge of the conjugate base is distributed over both nitrogen and oxygen.
- stabilization of adjacent carbonium ions (carbocations)

- The nitrogen of the amine donates its lone electron pair to the adjacent carbonium ion (carbocation).
- effect of substituents on basicity of aromatic amines
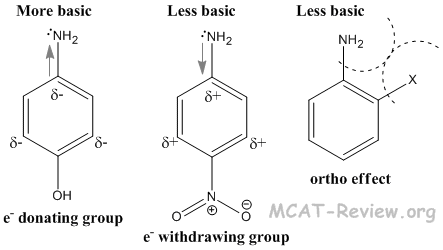
- Aromatic amines are weaker bases than aliphatic amines. This is because the amine donates its electron density to the aromatic ring. Also, the amine forms stable resonance structures with the aromatic ring, which is absent once the amine becomes protonated.
- Electron donating groups on the aromatic amine increase the basicity of aromatic amines. This is because the electron donating groups contribute to the electron density on the nitrogen.
- Electron withdrawing groups on the aromatic amine decrease the basicity of aromatic amines. This is because the electron withdrawing groups steal electron density from the nitrogen.
- Anything ortho to the amine, no matter whether it is electron donating or withdrawing, will decrease the basicity of the aromatic amine. This is because of the ortho effect, which is basically sterics. The protonated amine will have a greater steric interaction with the ortho group, so it will be less stable.
|
|
