|
|
Description
- nomenclature
- Aldehyde suffix: -al, -aldehyde.
- Ketone prefix: keto-, oxo-.
- Ketone suffix: -one, ketone.
- physical properties
- C=O bond is polar, with the carbon partially positive and oxygen partially negative.
- Dipole-dipole interactions give these molecules higher boiling points than their corresponding alkanes, but not as high as the corresponding alcohols or carboxylic acids.
- infrared absorption of C=O bond: 1700 cm-1
Important reactions
- nucleophilic addition reactions at C=O bond
- acetal, hemiacetal
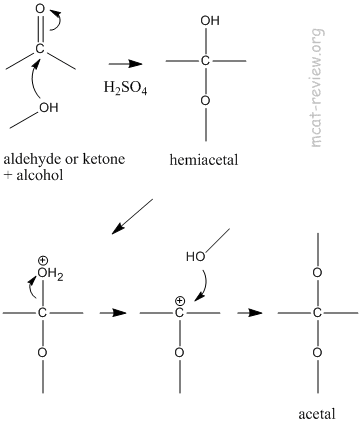
- Aldehydes and ketones react with 1 equivalent of alcohols to make hemiacetals.
- Aldehydes and ketones react with 2 equivalent of alcohols to make acetals.
- Hemiketal and ketal are the same as acetals except the starting compound must be a ketone and not an aldehyde. This is an old naming scheme that is no longer used.
- imine, enamine
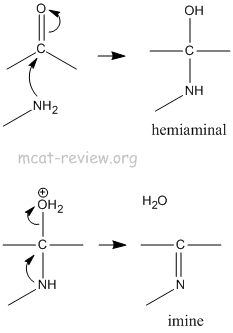
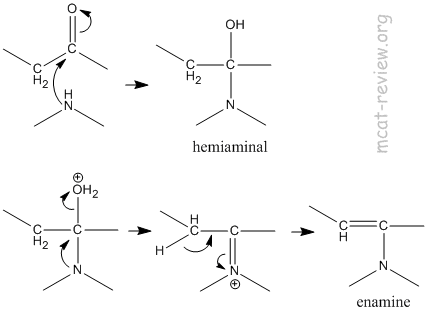
- Primary amine + aldehyde or ketone = imine.
- Secondary amine + aldehyde or ketone = enamine.
- reactions at adjacent positions
- haloform reactions
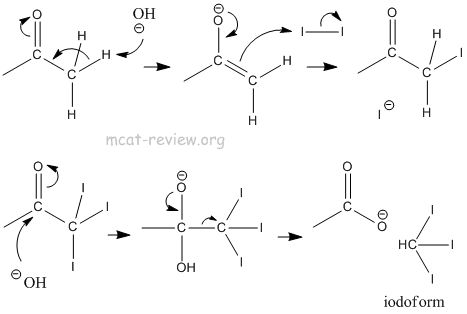
- Ketones + halogen = halogenation of the alpha position (carbon adjacent to the C=O group).
- Methyl ketone + halogen = haloform + carboxylate.
- Trihalogenated methyl = good leaving group.
- aldol condensation
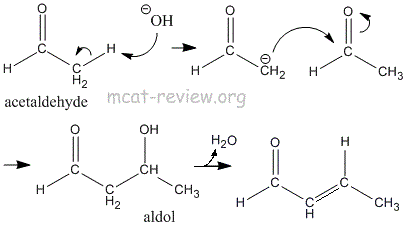
- Occurs because of the acidic alpha proton.
- 2 acetaldehyde -> aldo.
- Works for carbonyl compounds with an acidic alpha proton.
- oxidation: aldehydes oxidize to carboxylic acids. Ketones do not oxidize further.
- 1,3-dicarbonyls: internal H-bonding
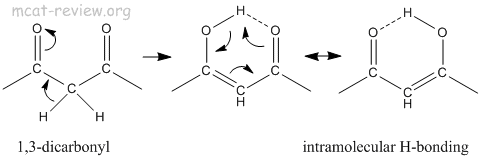
- 1,3-dicarbonyls have 2 carbonyl groups flanking a carbon atom with an acidic proton.
- Also referred to as active methylene compounds.
- Tautomerism causes one of the carbonyls to switch to its enol form, which contains an -OH group that hydrogen bonds with the other carbonyl C=O group on the same molecule. This is called intramolecular (internal) hydrogen bonding.
- keto-enol tautomerism
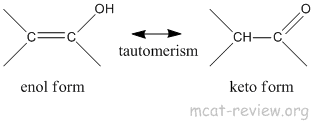
- Enol form is the one with the alcohol.
- Keto form is the one with the ketone.
- Keto form is more stable, it is the predominant form.
- organometallic reagents
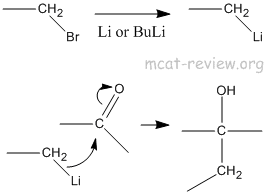
- Organometallic compounds makes R-, which attacks C=O to make R-C-OH.
- The purpose of organometallic compounds is to make carbon-carbon bonds.
- R-X + Li -> R-Li (byproduct: LiX)
- R-X + BuLi -> R-Li (byproduct: Bu-X)
- R-Li + C=O -> R-C-OH
- Wolff-Kishner reaction: reduces C=O to -CH2-
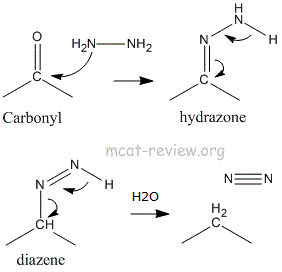
- C=O + NH2NH2 -> -CH2- + N2
- Grignard reagents
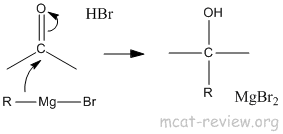
- Grignard reagents are just like organometallic reagents, they produce R-.
- R-X + Mg -> R-Mg-X
- R-Mg-X + C=O -> R-C-OH
General principles
- effect of substituents on reactivity of C=O; steric hindrance: bulky groups on either side of C=O blocks access to the electrophilic carbon, so reactivity goes down.
- acidity of alpha H; carbanions
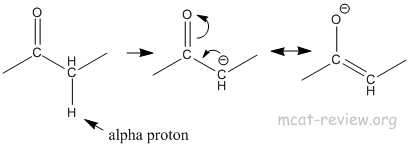
- Alpha proton is acidic because the resulting carbanion is stabilized by resonance.
- alpha, beta-unsaturated carbonyls - resonance structures
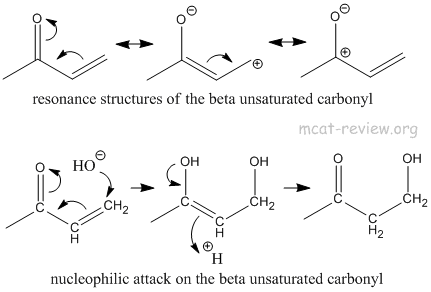
- α,β-unsaturated carbonyl + nucleophile -> addition of the nucleophile at the β position.
- Nucleophile attacks the beta carbon, pushing the α,β-unsaturated carbonyl into the enol form, which tautomerizes to the original carbonyl.
Old AAMC topics
- acetoacetic ester syntheses (this topic has been moved to the keto acids and esters section)
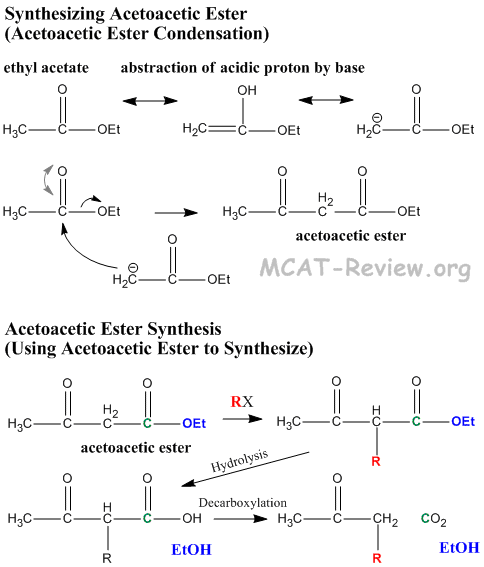
- Acetoacetic ester is synthesized by Claisen condensation of ethyl acetate in a process called acetoacetic ester condensation
- 2 x ethylacetate → ethyl acetoacetate
- acetoacetic ester = β-keto ester
- Claisen condensation = 1. alpha proton of ester leaves, 2. the resulting carbanion attacks the carbonyl group of another ester molecule, 3. Carbonyl group reforms and kicks off the alcohol group.
- "Acetoacetic ester synthesis" is a reaction where acetoacetic ester is used to synthesize a new ketone.
- Acidic alpha proton comes off, resulting carbanion attacks new R group.
- Hydrolysis of ester turns it into a β-keto carboxylic acid.
- β-keto acids undergo decarboxylation because the β-keto group stabilizes the resulting carbanion via enol formation. Enol converts back to keto form, and the net result of this reaction is that an R group is made to attach to the α carbon of acetone.
|
|
