|
|
Description
- nomenclature
- Prefix: hydroxyl, hydroxy.
- Suffix: -ol, alcohol.
- physical properties
- Hydrogen bonding.
- Higher boiling point than the same compound without the alcohol group.
- Water soluble as long as molecule does not contain a long hydrophobic region.
- infrared absorption of OH group: 3300 cm-1 and broad due to hydrogen bonding.
Important reactions
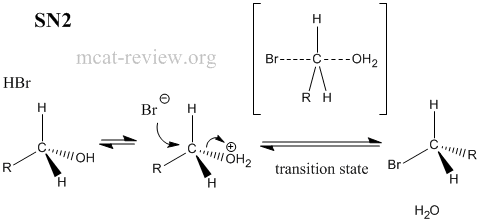
- substitution reactions: SN1 or SN2, depending on alcohol and derived alkyl halide
- R-OH + HX <--> R-X + H2O
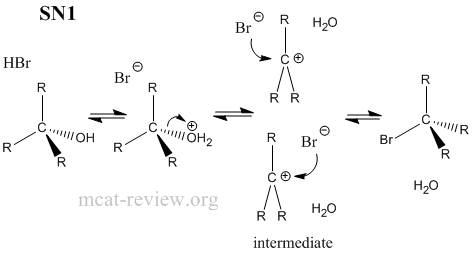
- Factors that favor sn1: stable carbocation, tertiary carbon center, protic solvent.
- Factors that favor sn2: unstable carbocation, primary carbon center, aprotic (but polar) solvent.
- All substitution reactions need a good leaving group.
- SN1 = unimolecular reaction, intermediate carbocation formed.
- SN2 = bimolecular reaction, passes through transition state.
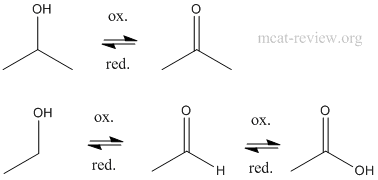
- oxidation
- KMnO4 and CrO3 will oxidize primary alcohols to carboxylic acids, but PCC (Pyridine Chlorochromate) and other weak oxidizing agents will only oxidize a primary alcohol to the aldehyde.
- Secondary alcohols always oxidize to the ketone.
- Tertiary alcohols do not oxidize.
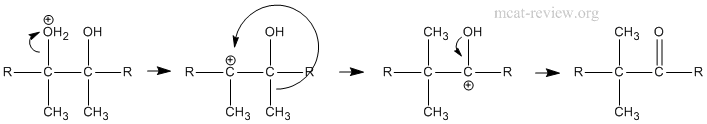
- pinacol rearrangement in polyhydroxyalcohols; synthetic uses

- Mechanism of pinacol rearrangement:
- protection of alcohols: the best protecting group for alcohol is the trimethylsilyl group.
- To protect, add Cl-SiMe3 to R-OH.
- The alcohol gets "capped" into R-O-SiMe3.
- To deprotect, add F-.
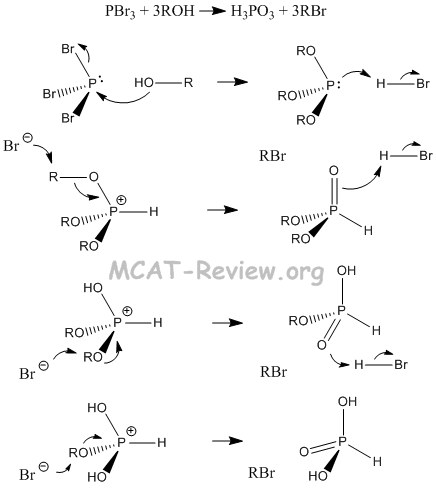
- reactions with SOCl2 and PBr3
- R-OH + SOCl2 --> R-Cl (by products: SO2 + HCl)
- R-OH + PBr3 --> R-Br (by products: H3PO3, R3PO3, HBr)
- preparation of mesylates and tosylates
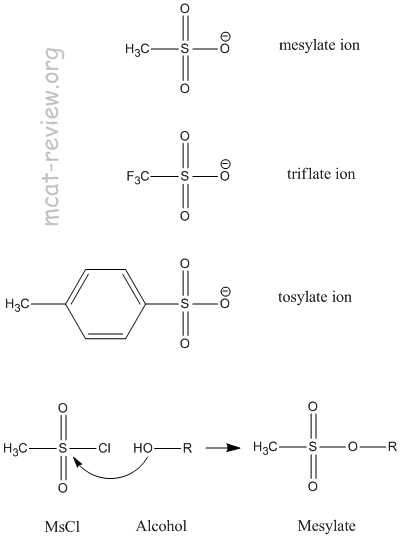
- Sulfonates R-SO3- are good leaving groups.
- The R can be:
- Methane, which makes methanesulfonate.
- Toluene, which makes tosylate.
- Trifluoromethane, which makes triflate.
- Mesylates can be prepared by reacting an alcohol (R-OH) with mesyl chloride (MsCl).
- Tosylates can be prepared by reacting an alcohol (R-OH) with tosyl chloride (TsCl).
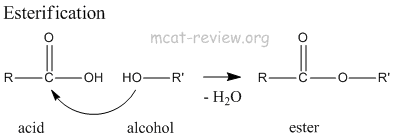
- esterification: acid + alcohol = ester
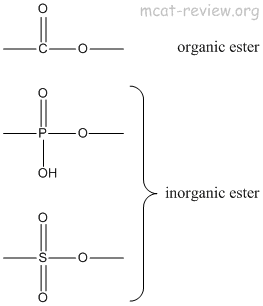
- inorganic esters: replace the carbon of esters with a different atoms.
- Reactions involving the formation of inorganic esters:
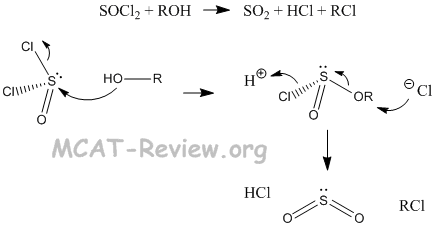
- Formation of mesylates and tosylates are also reactions that involve inorganic esters.
- In biochemistry DNA/RNA polymerization, the 3'-OH alcohol group attacks the 5'-phosphate to form an inorganic ester linkage (phosphodiester linkage of DNA/RNA backbone).
General principles
- hydrogen bonding: hydrogen bonding in alcohols give them a higher boiling point than their corresponding alkanes.
- acidity of alcohols compared to other classes of oxygen-containing compounds: lower pKa = more acidic.
| Compound |
pKa |
| COOH (carboxylic acids) |
5 |
| ArOH (phenols) |
10 |
| H2O (water) |
16 |
| ROH (alcohols) |
16 |
| -CH2(CO)-R (alpha hydrogen in aldehydes and ketones) |
20 |
| -CH2(CO)-OR (alpha hydrogen in esters) |
25 |
- effect of chain branching on physical properties: going from straight chain to branched alkane (with same # carbons) = higher freezing/melting point, lower boiling point.
|
|
