|
|
Aliphatic - alkanes
- Description
- nomenclature
| # C atoms |
Name for straight chain alkane |
Name for cyclic alkane |
| 1 |
Methane |
N/A |
| 2 |
Ethane |
N/A |
| 3 |
Propane |
Cyclopropane |
| 4 |
Butane |
Cyclobutane |
| 5 |
Pentane |
Cyclopentane |
| 6 |
Hexane |
Cyclohexane |
| 7 |
Heptane |
Cycloheptane |
| 8 |
Octane |
Cyclooctane |
| 9 |
Nonane |
Cyclononane |
| 10 |
Decane |
Cyclodecane |
- After Decane, there is Undecane (11), Dodecane (12), Tridecane (13), Tetradecane (14), and so forth for eleven membered alkanes upwards.
- physical properties
- Hydrophobic.
- London Dispersion Forces present only.
- Lower boiling points than compounds the same size but with functional groups.
- Very long alkanes can have very high boiling points due to the sum of all the dispersion forces. A useful reference is that heptane, the 7 membered alkane, has the same boiling point as water.
- Important reactions
- combustion
- Complete combustion of alkanes: alkane or cycloalkane + O2 → CO2 + H2O
- Complete combustion of anything: fuel + oxygen → carbon dioxide + water
- substitution reactions with halogens, etc.
- Alkane + halogen + free radical initiator → alkyl halide
- Free radical initiators = hν (UV light) or peroxides.
- Substitution occurs via a free radical mechanism - see below.
- General principles
- stability of free radicals; chain reaction mechanism; inhibition
- The more substituted the radical, the more stable it is.
- Stability: 3° > 2° > 1° > methyl.
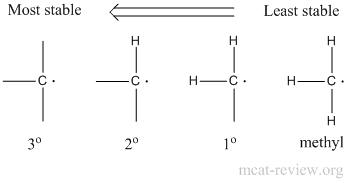
- Substitution will occur preferentially at the more substituted carbon atom.
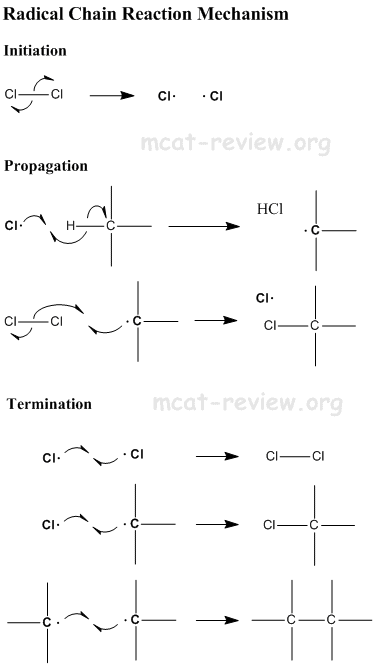
- The free radical chain reaction is dependent on the presence of free radicals. Therefore, anything that inhibits free radicals will inhibit this reaction. One example is antioxidants, which eats up free radicals and therefore inhibits the free radical chain reaction.
- ring strain in cyclic compounds
- Cyclopropane has the highest ring strain.
- Cyclobutane has the second highest ring strain.
- Cyclohexane has the lowest ring strain.
- Any ring with greater or equal to 14 carbon atoms has the next lowest ring strain.
- Stick with the above rule and you can answer any questions comparing ring strain. The MCAT will not require you to make weird ring strain comparisions, for example between cyclopropane and cycloheptane.
- Ring strain consists of Angle (Baeyer) strain and Torsional strain.
- Angle (Baeyer) strain is caused by deviation from the ideal sp3 tetrahedral bond angle of 109.5°
- Torsional strain is caused by the molecule having eclipsed conformations instead of staggered ones.
- Cyclopropane has both angle (Baeyer) strain and torsional strain.
- Cyclohexane, in the chair conformation, has no angle (Baeyer) or torsional strain.
- You'll frequently see people write Bayer strain instead of Baeyer strain. They mean the same thing.
- bicyclic molecules
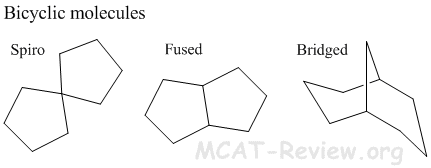
- Bicyclic molecules have more ring strain than monocyclic molecules. Except for spiro bicyclics, which have similar ring strain as their monocyclic counterparts.
|
|
