|
|
sigma and pi bonds
- ... sigma and pi bonds is a repeat of general chemistry bonding
- hybrid orbitals (sp3, sp2, sp and respective geometries)
- valence shell electron pair repulsion (VSEPR) theory, prediction of shapes of molecules (e.g., NH3, H2O, CO2)
- structural formulas for molecules involving H, C, N, O, F, S, P, Si, Cl
|
# valence e- |
Usual # bonds |
Typically found in |
| H |
1 |
1 |
Hydrocarbon (alkane, alkene, alkyne), hydride. All organic compounds contain hydrogen. |
| C |
4 |
4 |
Alkane, alkene, alkyne, aromatic rings. All organic compounds contain carbon. |
| N |
5 |
3 |
Amine, amide, imine, hydrazone, oxime, nitro compound, diazo compound, nitrile/cyanide, azide |
| O |
6 |
2 |
Alcohol, ether, aldehyde, ketone, carboxylic acid, acyl halide, anhydride, amide, ester, ozone |
| F |
7 |
1 |
Fluoride |
| S |
6 |
2 or 6 |
Thiol, sulfide, sulfate, sulfite |
| P |
5 |
3 or 5 |
Phosphorous compound, phosphate, phosphite |
| Si |
4 |
4 |
Silane, silicon dioxide |
| Cl |
7 |
1 |
Chloride, hypochlorite, chlorite, chlorate, perchlorate |
- H and C
- N
- O
- F
- S
- P
- Si
- Cl
- delocalized electrons and resonance in ions and molecules
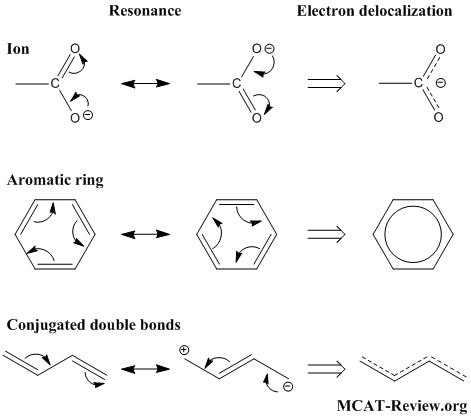
- Resonance structures result from electrons not being fixed in position (that's why you "push" electrons when drawing resonance structures).
- When electrons are not fixed in position, they are delocalized electrons.
- For all practical purposes, resonance and electron delocalization mean the same thing.
- In ions, resonance and electron delocalization occurs to "distribute" the charge around.
- In molecules, resonance and electron delocalization occurs in aromatic rings and conjugated double bonds.
Multiple bonding
- its effect on bond length and bond energies
- Multiple bonding decreases bond length.
- Multiple bonding increases bond energy.
- rigidity in molecular structure
- Multiple bonding increases rigidity in molecular structure.
- Single bonds can rotate, but double and triple bonds can't.
- Even partial double bonds like those found in the peptide bond prevents free rotation.
Stereochemistry of covalently bonded molecules
isomers
- Same molecular formula, different structural formula.
- "Same in writing, different in drawing..."
structural isomers
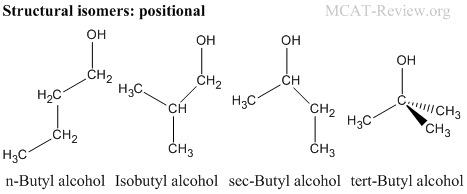
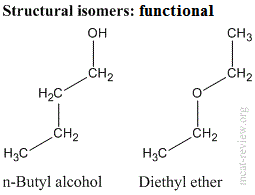
- Structural (constitutional) isomers have the same molecular formula, but different connectivity.
- Positional isomers: structural isomers that have the same functional groups positioned differently.
- Functional isomers: structural isomers that have different functional groups.
geometric isomers
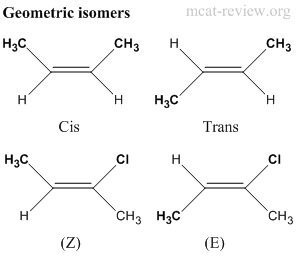
- Geometric isomers have the same molecular formula, same connectivity, but have different orientation across a double bond.
- When both sides of the double bond contains the same 2 groups, then cis and trans is used.
- Cis = same side, Trans = opposite sides.
- When different groups are attached to either side, Z and E is used.
- Z is when the higher priority groups (ranked according to the Cahn-Ingold-Prelog rules) are orientated on the same side across the double bond. Zusammen is the German word for together.
- E is when the higher priority groups are orientated on different sides across the double bond. Entgegen is the German word for opposed.
stereoisomers (e.g. diastereomers, enantiomers, cis/trans isomers)
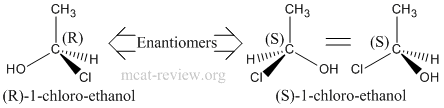
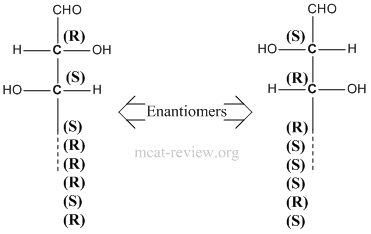
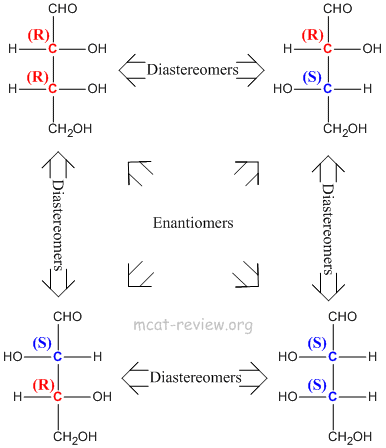
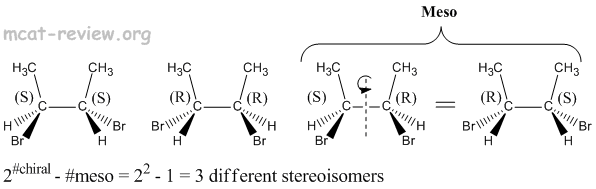
- Stereoisomers have the same molecular formula, same connectivity, but have different 3-D arrangements across one or more asymmetric (chiral) centers.
- Chiral center is any atom with 4 different entities attached to it.
- Enantiomers are mirror images of each other. That means ALL chiral centers in one enantiomer is reversed in the other.
- You can't have stereoisomers if you don't have a chiral center.
- Diastereomers - more than one chiral center, inversion of stereochemistry on some but not all of its chiral centers. For examples, diastereomers would have stereochemistries of (R)-(R) vs (R)-(S). Another example of diastereomers would be (R)-(R)-(S)-(R) vs (R)-(R)-(R)-(R).
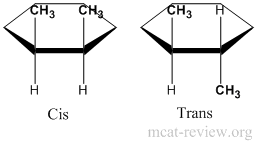
- In rings, it is easier to assign stereoisomers as cis/trans rather than R or S. Cis is having the same groups on the same side of the ring. Trans is having the same groups on different sides of the ring.
- A compound will have a total of 2#chiral centers stereoisomers if it is not meso.
- Meso compounds may have chiral centers, but as a molecule, they are achiral and optically inactive.
- Meso compounds reduce the total number of stereoisomers.
- Stereoisomers have the same chemical properties.
- Enantiomers have the same physical properties.
- Diastereomers have different physical properties.
- Note: in biological molecules, people use D and L for R and S, respectively.
- Caution: D and L (absolute configurations) are NOT the same as d and l (relative configuration). Read the section below on rotation of polarized of light for more details.
conformational isomers
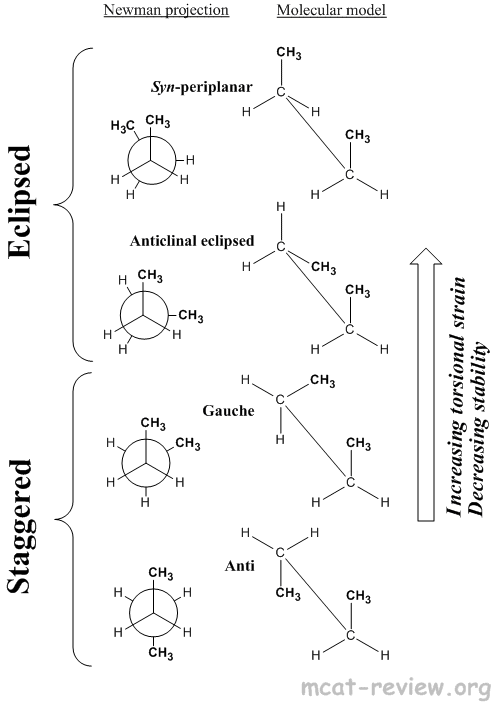
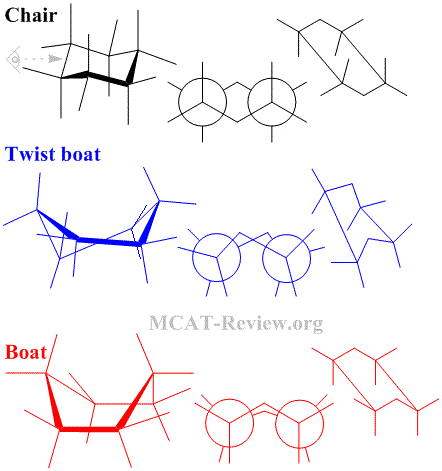
- Conformational isomers have the same molecular formula, same connectivity, same stereochemistry, but can rotate about a single bond to switch between different conformations.
- Technically, conformational isomers are not really isomers because you don't have to break any bonds to convert from one conformation to another. They are more accurately called conformers.
- Conformers about a single bond
- Eclipsed
- Syn-periplanar: highest torsional strain, most unstable, bulky groups eclipse each other.
- Anticlinal eclipsed: high torsional strain, unstable, bulky groups eclipse hydrogens.
- Staggered
- Gauche: low torsional strain, stable, bulky groups 60° staggered.
- Anti: lowest torsional strain, most stable, bulky groups 180° staggered.
- Single bonds will rotate such that it achieves the most stable conformation.
- Conformers of cyclohexose
- Chair: most stable, everything is staggered.
- Twist boat: less stable, things are not completely eclipsed.
- Boat: least stable, everything is eclipsed.
- Hexose rings will twist and turn to achieve the most stable conformation.
- Torsional strain: the strain due to eclipsing of groups across a single bond.
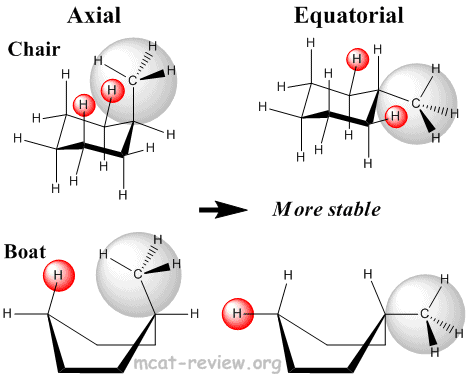
- Steric interactions
- Axial: most unstable because the axial groups are orientated with a high degree of clashing.
- Equatorial: most stable because the equatorial groups are orientated away from one another.
- Bulky groups like to be in the equatorial position.
- Most stable conformation: completely staggered (chair), with bulky groups in the equatorial position.
- Least stable conformation: completely eclipsed (boat), with bulky groups in the axial position.
polarization of light, specific rotation
- Light is an electromagnetic wave.
- Electromagnetic waves are waves of electric and magnetic fields (in phase, but perpendicular to each other and also to the direction of propagation).
- Normal light has the EM fields in all directions (in a 360° circle perpendicular to the direction of propagation).
- Polarized light has EM fields all in one direction.
- Specific rotation: chiral molecules containing a single enantiomer will rotate polarized light (to varying degrees) either to the left or to the right. This is why chiral molecules are said to be "optically active".
- Left rotation: (-) or l or levorotatory.
- Right rotation: (+) or d or dextrorotatory.
- Caution: (+) or (-) does NOT correspond to R/S configurations.
- Caution: d and l is NOT the same as D and L. The upper case letters denote absolute configurations in sugars.
absolute and relative configuration
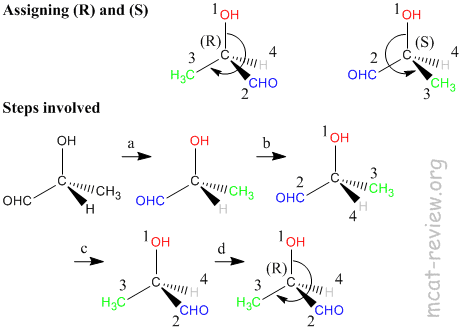
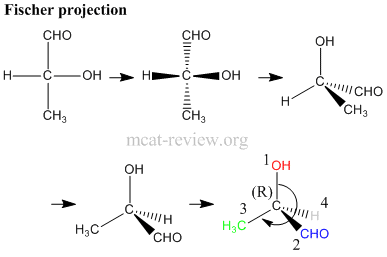
- Steps in assigning (R) and (S) - refer to figure above.
- a. Is the carbon center chiral? For our molecule, the answer is yes because 4 different groups are attached to the carbon atom.
- b. Assign priorities according to the Cahn-Ingold-Prelog rules (see below).
- c. Turn the molecule such that the lowest priority group is at the back.
- d. Rotate from the 1st to 2nd to 3rd priority group like a steering wheel. It's (R) if you end up turning right, and it's (S) if you end up turning left.
- note: if you're good at visualizing stuff, you do this much faster by skipping step c.
- Absolute configuration is the (R) or (S) that's labeled on the chiral centers.
- Relative configuration is always defined in relationship to another chiral center. The direction that a molecule rotates plane-polarized light is the prime example of relative configuration.
- Before the mid-1800s, people did not have an understanding of the tetrahedral carbon atom, so they did not have absolute configurations. Instead, they used the relative configurations of which way a compound rotates plane-polarized light.
- The definition for relative configuration can be very broad. For example, you may arbitrarily assign one chiral center to be R* (even though it may or may not actually be R) as long as it is of opposite configuration to S* (which may or may not actually be S). Additionally, the cis or trans configuration that describes how one group is orientated relative to another group is also an example of relative configuration. Reactions can also proceed via retention or inversion, which describes the stereochemistry in relationship with the original reactant.
| Configuration |
Notation |
| Absolute (R) |
R, D |
| Absolute (S) |
S, L |
| Relative (rotate light right) |
+, d |
| Relative (rotate light left) |
-, l |
conventions for writing R and S forms
- If only 1 chiral center
- (R/S)-molecule, where R/S is the absolute configuration and molecule is the name of the compound.
- For example, (R)-2-hydroxyl-propanal.
- If more than 1 chiral center
- (#R/S, #R/S)-molecule, where # is the carbon number (in ascending order), R/S is the absolute configuration, and molecule is the name of the compound.
- For example, (2R,3S)-2,3,4-hydroxyl-butanal.
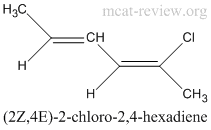
conventions for writing E and Z forms
- If only 1 double bond
- (E/Z)-molecule, where E/Z is the geometric configuration across the double bond, and molecule is the name of the compound.
- For example, (Z)-2-chloro-2-butene. (see geometric isomer figure)
- If more than 1 double bond
- (#E/Z, #E/Z)-molecule, where # is the carbon number (the smaller one in the double bond, in ascending order), and molecule is the name of the compound.
Cahn-Ingold-Prelog rules for assigning priority
- Start with shell 1, which is the atoms directly bonded to the chiral carbon.
- The atom with the higher MW has greater priority.
- If atoms are the same, look at next shell.
- Shell 2, which are the atoms adjacent around shell 1 atoms.
- The atom with the higher MW has greater priority.
- If same atoms, the more # of the high MW species, or the more bonds to the high MW species wins.
- For example, -CHO will have higher priority than -CH2OH because the aldehyde has a double bond to oxygen.
- For example, -CH(OH)2 will have higher priority than -CH2OH because the diol has 2 oxygens while the alcohol only has 1.
- What about -CH(OH)2 vs. CH2F? Ans: It doesn't matter how many oxygens there are, because fluorine has greater molecular weight. So fluorine has higher priority.
- If by now, everything is still the same, go to shell 3 and repeat the procedure.
racemic mixtures, separation of enantiomers by biological means
- Racemic mixtures contain equal amounts of both enantiomers. Another name for racemic mixtures is racemate.
- Racemic mixtures do not rotate polarized light, so they are optically inactive.
- Separation of enantiomers
- Convert enantiomers to diastereomers.
- Separation of diastereomers.
- Convert diastereomers back to enantiomers.
- Separation of enantiomers by biological means
- Enzymes are highly specific and can differentiate between enantiomers. For example, if an enzyme digests or modifies all L-amino acids, then you'd be able to use that enzyme to separate a D/L racemic mixture.
- In nature, all proteins are made up of L-amino acids.
|
|
