|
|
Description; nomenclature
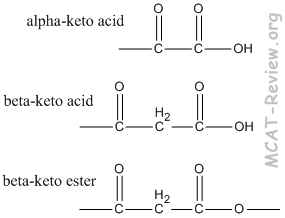
- α-keto acid = 2-oxo acid. For example: α-ketopropanoic acid = 2-oxopropanoic acid
- β-keto acid = 3-oxo acid.
- α-keto ester = 2-oxo ester.
- β-keto ester = 3-oxo ester. For example: methyl β-ketobutanoate = methyl 3-oxobutanoate
Important reactions
- decarboxylation
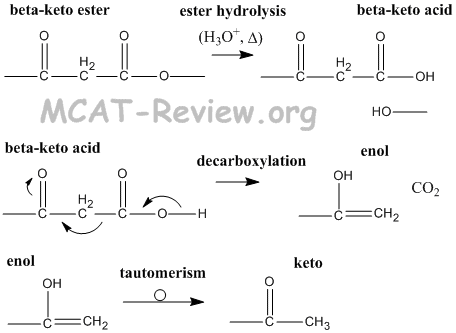
- beta-keto esters → beta-keto acids → enols → ketos
- decarboxylation of beta-keto acids is facile because the enol stabalizes the reaction intermediate.
- acetoacetic ester synthesis
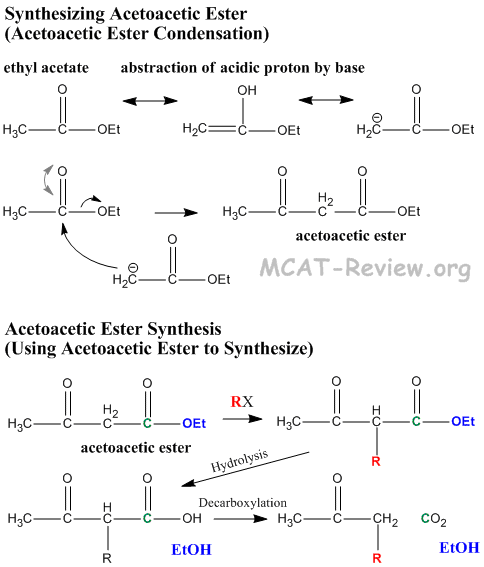
- Acetoacetic ester is synthesized by Claisen condensation of ethyl acetate in a process called acetoacetic ester condensation
- 2 x ethylacetate → ethyl acetoacetate
- acetoacetic ester = β-keto ester
- "Acetoacetic ester synthesis" is a reaction where acetoacetic ester is used to synthesize a new ketone.
- Acidic alpha proton comes off, resulting carbanion attacks new R group.
- Hydrolysis of ester turns it into a β-keto carboxylic acid.
- β-keto acids undergo decarboxylation because the β-keto group stabilizes the resulting carbanion via enol formation. Enol converts back to keto form, and the net result of this reaction is that an R group is made to attach to the α carbon of acetone.
General principles
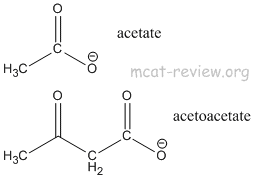
- acidity of alpha hydrogen and beta-keto ester
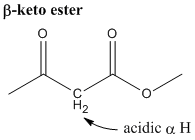
- Any hydrogen alpha to (adjacent to) a carbonyl group is more acidic than a regular hydrogen. The alpha hydrogen of a beta-keto ester is even more acidic because it's adjacent to 2 carbonyl groups.
- The reason for the acidity is the stabilization of the deprotonated species by the enolate ion resonance structures.
- keto-enol tautomerism
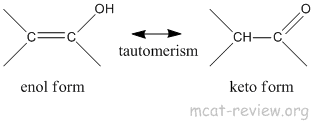
- enol ends with an "ol" so it has an alcohol group.
- keto is a ketone group.
- enol → keto because the keto form is more stable.
- You can change a ketone to an enolate ion by abstracting the alpha hydrogen. However, that's not tautomerism.
|
|
