|
|
Description
- nomenclature
- Suffix: -oic acid, carboxylic acid, -dioic acid.
- physical properties and solubility
- High boiling point due to hydrogen bonding.
- Soluble in water.
- infrared absorption
- C=O at 1700 cm-1
- -OH at 3100 cm-1
Important reactions
- carboxyl group reactions
- nucleophilic attack
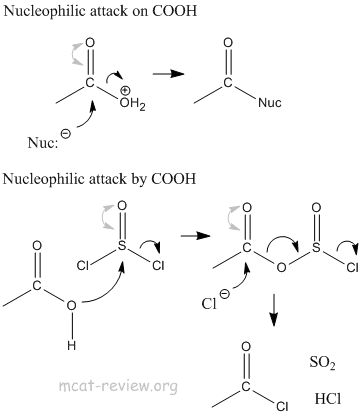
- Nucleophilic attack occurs on the electrophilic carbon of C=O.
- Nucleophilic attack occurs by the nucleophilic oxygen of COOH.
- reduction
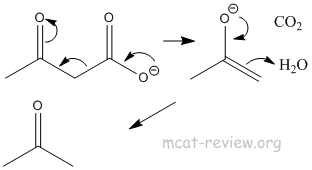
- decarboxylation: occurs for beta-keto acids
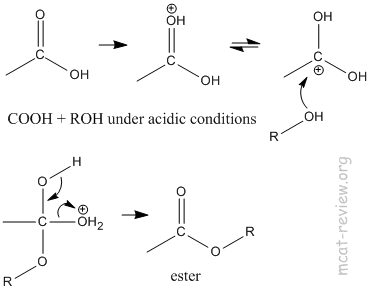
- esterification: COOH + ROH under acidic conditions = ester.
- reactions at 2 position
- halogenation: RCOOH + X2 -> halogenation at the alpha carbon (2 position).
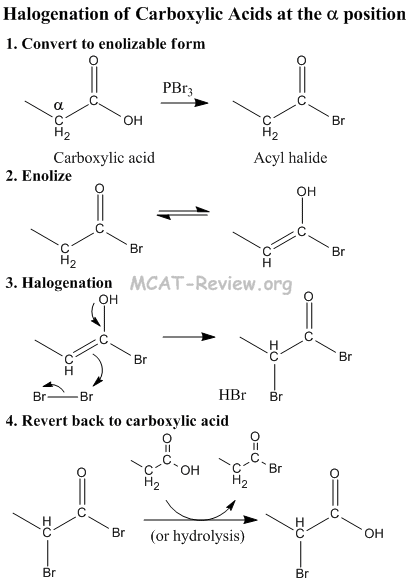
- substitution reactions: RCOOH + E+ -> substitution at the alpha carbon (2 position).
- Carboxylic acid converted to Acyl Halide, which can enolize.
- Acyl Halide tautomerizes to its enol form by abstraction of acidic alpha hydrogen.
- Halogen (or some other E+) gets attacked by alpha position.
- Revert back to carboxylic acid. The net effect is that the alpha H get substituted by an electrophile.
General principles
- H bonding: COOH has high boiling point because of H bonding.
- dimerization: Hydrogen bonding causes dimerization of carboxylic acids.
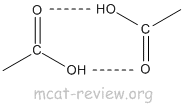
- acidity of the carboxyl group: pKa of COOH is about 5. pKa of H+ is 0 while the pKa of water is 16. So, COOH can be classified as a weak acid. Vinegar is dilute acetic acid, which is CH3COOH.
- inductive effect of substituents: electron withdrawing groups makes the acid stronger.
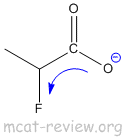
- electron withdrawing groups attached to positions close to the COOH helps to distribute the charge of the COO- and stabilize it.
- A more stabilized carboxylate ion makes a stronger acid.
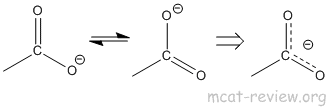
- resonance stability of carboxylate anion: the reason why COOH is a good acid is because the conjugate base (carboxylate ion) is stabilized by resonance.
|
|
