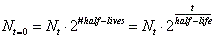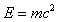|
|
Atomic Structure and Spectra
Emission spectrum of hydrogen (Bohr model)
- Bohr model:
- An electron orbits the positively charged nucleus in the same way that the earth orbits the Sun.
- Electrostatic attraction pulls the electron toward the nucleus.
- The electron orbits at high speed to prevent it from crashing into the nucleus.
- The electron can orbit at different energy levels: n=1, n=2, n=3 ...etc.
- The higher the energy level, the larger the radius from the nucleus.
- Emission spectrum of hydrogen:
- When an electron transitions from a higher energy level to a lower energy level, it emits electromagnetic radiation.
- The emission spectrum of hydrogen consists of sharp, distinct lines.
Atomic energy levels
- quantized energy levels for electrons
- The distinct lines of the emission spectrum prove that electron energy is quantized into energy levels.
- If electron energy is not quantized, then a continuous spectrum would be observed.
- The energy of the energy levels is governed by:
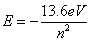 , where E is energy and n is the energy level. , where E is energy and n is the energy level.
- The equation is negative, so all energies are negative.
- Negative energies mean that it is energy that contributes to the "stability" of the system - the electron binding energy.
- The more negative (lower) the energy, the more stable the orbit, the harder it is to knock out the electron.
- The less negative (higher) the energy, the less stable the orbit, the easier it is to knock out the electron.
- At the highest energy, 0 eV, there is no binding energy, so the electron dissociates.
- For atoms other than hydrogen, the shape of the energy level curve stays the same. However, the numerator is a constant other than 13.6 eV.
- The precise relationship for atoms other than hydrogen is:
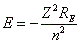 , where Z is the atomic number. , where Z is the atomic number.
- Higher Z values give more negative binding energy (more stable) because the more charge, the more electrostatic attraction.
- calculation of energy emitted or absorbed when an electron changes energy levels
- The wavelength of the emitted or absorbed radiation is governed by the Rydberg formula:
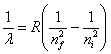 , where lambda is the wavelength, nf is the final energy level, ni is the initial energy level, and R is the rydberg constant. , where lambda is the wavelength, nf is the final energy level, ni is the initial energy level, and R is the rydberg constant.
- The energy of the emitted or absorbed radiation is:
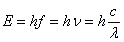 , where E is energy, f and v both mean frequency and c is the speed of light. , where E is energy, f and v both mean frequency and c is the speed of light.
- Energy is emitted for transitions to lower energy levels (nf < ni).
- Energy is absorbed for transitions to higher energy levels (nf > ni).
Atomic Nucleus
Atomic number, atomic weight
- Atomic number = the number of protons.
- The atomic number is what defines an element.
- When two things have the same number of protons, they are the same element.
- Atomic weight = the weighted average of atomic mass for all isotopes of a given atom.
- Atomic mass = number of protons + neutrons.
- The atomic mass is used for an isotope.
- The atomic weight is used for an element.
- In standard notation the atomic number is always at the bottom, and the weight is always on top:
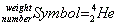
- An easy way to remember this is that the atomic number is "fundamental" to the identity of the element, so it is located at the foundation.
Neutrons, protons, isotopes
- Neutrons = neutral particles that reside in the nucleus.
- Protons = positive particles that reside in the nucleus.
- Isotopes = things with the same number of protons, but different number of neutrons.
| Atomic particles
| | Name |
Mass (amu) |
Charge |
Location |
| Proton |
1 |
+1 |
In the nucleus |
| Neutron |
1 |
0 |
In the nucleus |
| Electron |
0 |
-1 |
Surrounding the nucleus |
- Nucleons = protons or neutrons.
Isotopes
- When two things have the same number of protons but different number of neutrons, they are isotopes of the same element.
- Isotopes often have similar chemical properties, but different stabilities (some decay and give off radiation, some don't).
Nuclear forces
- Two forces are at work in the nucleus: the strong force and the electromagnetic force.
- The strong force binds the nucleons together, and therefore contributes to the binding energy.
- The electromagnetic force is due to electrostatic repulsion between the positively charged protons in the nucleus.
- The nucleus stays together because the strong force is much stronger than the electromagnetic repulsion.
- The strong force is also called the "nuclear force".
... see forces section
Radioactive decay: alpha, beta, gamma, half-life, exponential decay, semi-log plots
- Alpha decay:
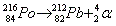 . Ejection of a helium nucleus at relatively low speed. . Ejection of a helium nucleus at relatively low speed.
- Beta decay:
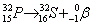 . Ejection of a high speed electron. . Ejection of a high speed electron.
- Gamma decay:
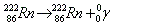 . Release of high energy electromagnetic wave. . Release of high energy electromagnetic wave.
| Name |
Notation |
Information |
| Alpha particle |
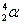 |
Weakest form of radiation. Can be stopped by a sheet of paper. It is essentially a relatively low speed helium nucleus. |
| Beta particle |
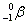 |
More energy than an alpha particle. Can be stopped by aluminum foil. It is a high speed electron. |
| Gamma ray |
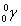 |
Strongest form of radiation. It is a high energy electromagnetic wave. Can be stopped by a thick layer of lead or concrete. |
- Some notes on α, β, and γ decay
- Conservation of mass dictates that total atomic weight before the decay equal the total atomic weight after.
- Conservation of charge dictates that the total atomic number before the decay equal the total atomic number after.
- Don't get thrown off by particles you do not recognize. As long as they have a weight and a charge, just incorporate these numbers in your calculations.
- MCAT problems on identifying decay products are just math work.
- Remember: the atomic number (the bottom number) determines what element it is.
- half-life is the time it takes for the amount of something to half due to decay.
- After 1 half-life, the amount of the original stuff decreases by half.
- After 2 half-lives, the amount of the original stuff decreases by a factor of 4.
- After 3 half-lives, the amount of the original stuff decreases by a factor of 8.
- The mathematical expression for this is:
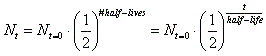 , where N sub t=0 is the amount the original starting material. N sub t is the amount of the original material that is still left. Lastly, t is time. , where N sub t=0 is the amount the original starting material. N sub t is the amount of the original material that is still left. Lastly, t is time.
- Although the above is the official half-life equation, people like to multiply rather than to divide. Therefore, a more user friendly equation is:
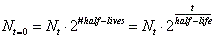
- Stability
- When something is stable, it doesn't decay.
- When something is unstable, it decays.
- The more unstable something is, the shorter the half-life.
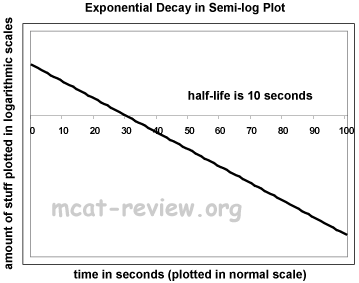
- Exponential decay:
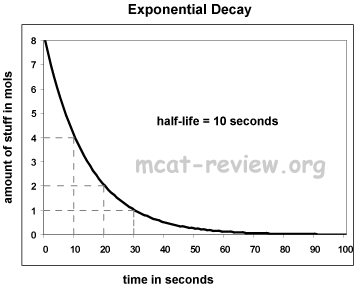
- Semi-log plots: for the purposes of the MCAT, semi-log plots convert exponential curves into straight lines.
- Something that curves up becomes a straight line with a positive slope.
- Something that curves down becomes a straight line with a negative slope.
- For exponential decay, a semi-log plot graphs the log of amount vs. time.
- For exponential decay, a semi-log plot is a straight line with a negative slope.
- The semi-log plot intercepts the x axis where the original y value is 1.
General nature of fission
- Fission = one nuclei splitting apart.
- Uranium undergoes fission when struck by a free neutron.
- The fission of uranium generates more neutrons, which goes on to split other Uranium nuclei. This is called a chain reaction.
General nature of fusion
- Fusion = two nuclei coming together.
- The Sun works by fusion.
- Hydrogen in the Sun fuses to form helium.
Mass deficit, energy liberated, binding energy
- Mnucleons = Matom + binding energy/c2
- Mnucleons > Matom because some of the Mnucleons is converted to binding energy that holds the nucleons together.
- Mnucleons = mass of all the nucleons that make up the atom in their free, unbound state.
- Matom = mass of the atom.
- Mnucleons - Matom = mass deficit (also called mass defect) = ΔM.
- Binding energy = converting ΔM into its equivalent in energy = ΔM c2.
- Energy liberated = binding energy.
- The conservation of mass and energy: the total mass and energy before a reaction is always the same as the total mass and energy after the reaction.
- If the total mass before the reaction is different from the total mass after the reaction, then the difference in mass is made up for by energy.
- The difference in mass before and after a reaction is called the mass deficit or mass defect.
- The energy that makes up for the mass deficit is calculated by:
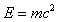
- Energy is liberated when mass is lost during a reaction.
- Energy is absorbed with mass is gained during a reaction.
- More notes on binding energy:
- Binding energy most commonly refers to nuclear binding energy (the energy that binds the nucleons together).
- Binding energy is due to the strong force. ...more on forces
- Binding energy per nucleon is strongest for Iron (Fe 56).
- Binding energy per nucleon is the weakest for Deuterium (the 2-nucleon isotope of hydrogen).
- Less commonly used is the electron binding energy. This is because electron binding energy is more commonly referred to as the ionization energy.
|
|

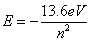 , where E is energy and n is the energy level.
, where E is energy and n is the energy level.
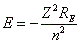 , where Z is the atomic number.
, where Z is the atomic number.
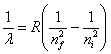 , where lambda is the wavelength, nf is the final energy level, ni is the initial energy level, and R is the rydberg constant.
, where lambda is the wavelength, nf is the final energy level, ni is the initial energy level, and R is the rydberg constant.
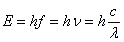 , where E is energy, f and v both mean frequency and c is the speed of light.
, where E is energy, f and v both mean frequency and c is the speed of light.
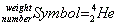
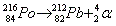 . Ejection of a helium nucleus at relatively low speed.
. Ejection of a helium nucleus at relatively low speed.
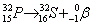 . Ejection of a high speed electron.
. Ejection of a high speed electron.
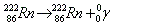 . Release of high energy electromagnetic wave.
. Release of high energy electromagnetic wave.
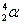
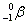
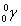
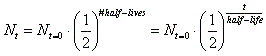 , where N sub t=0 is the amount the original starting material. N sub t is the amount of the original material that is still left. Lastly, t is time.
, where N sub t=0 is the amount the original starting material. N sub t is the amount of the original material that is still left. Lastly, t is time.